2025


2024

















2023











2022














2021










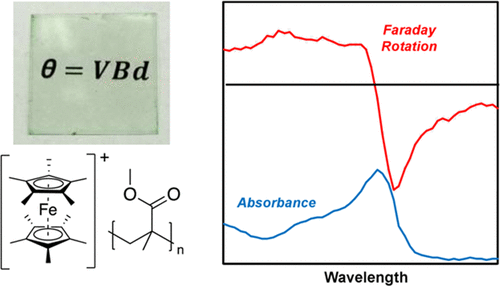







2020



















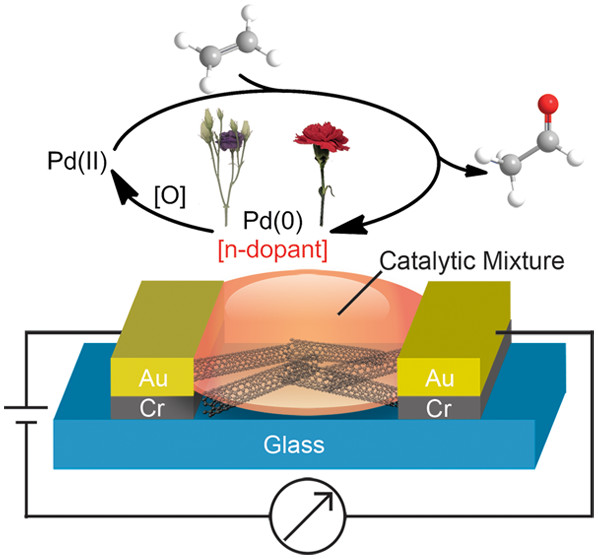

2019


















2018
2017
Nanomaterials that undergo a physical change upon chemical warfare agent (CWA) exposure can potentially be used in detectors to warn soldiers of their presence or in fabrics to provide on-demand protection. In this study, hybrid nanoparticles (NPs) were prepared by grafting a CWA-responsive polymer from a silicon dioxide (SiO2) surface using ring opening metathesis polymerization; the covalent functionalization of the polymers on the NP surface was confirmed by gel permeation chromatography, dynamic light scattering, and transmission electron microscopy analysis. The polymer-grafted SiO2 NPs were found to undergo a pronounced decrease (approximately 200 nm) in their hydrodynamic radius upon exposure to CWA simulants trifluoroacetic acid and diethyl chlorophosphate in toluene. This decrease in hydrodynamic radius is attributed to the electrophile-mediated ionization of the triarylmethanol responsive unit and represents a rare example of polycation formation leading to polymer chain collapse. We have ascribed this ionization-induced collapse to the formation of a favorable stacking interaction between the planar triarylcations. These studies have important implications for the development of breathable fabrics that can provide on-demand protection for soldiers in combat situations.
We report the synthesis and characterization of seven new linearly conjugated ladder compounds of the phenylene-containing oligoacene molecule class. Each derivative incorporates a fused four-membered-ring linkage in the acene-like backbone. Crystal packing, spectroscopic and electrochemical properties of the molecules are described.
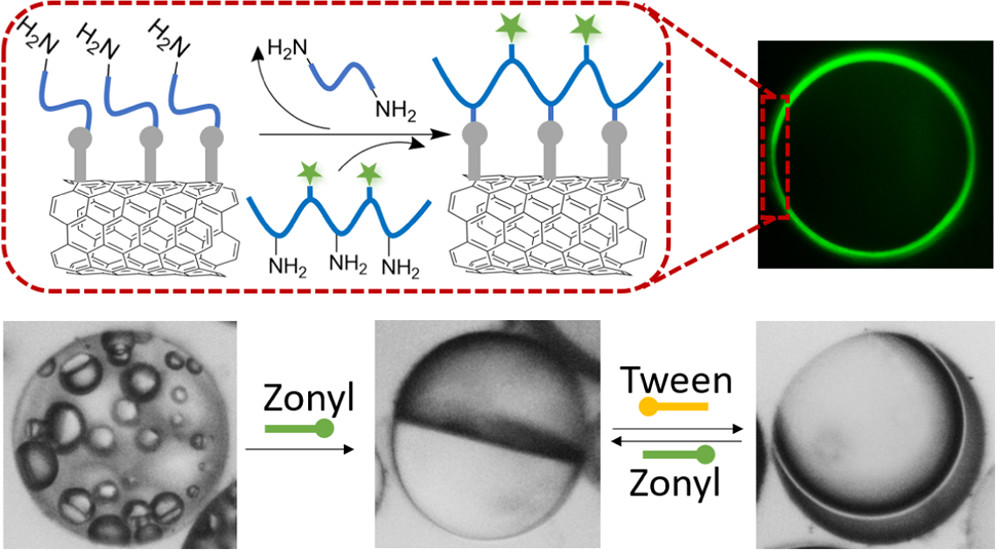
Complex emulsions, including Janus droplets, are becoming increasingly important in pharmaceuticals and medical diagnostics, the fabrication of microcapsules for drug delivery, chemical sensing, E-paper display technologies, and optics. Because fluid Janus droplets are often sensitive to external perturbation, such as unexpected changes in the concentration of the surfactants or surface-active biomolecules in the environment, stabilizing their morphology is critical for many real-world applications. To endow Janus droplets with resistance to external chemical perturbations, we demonstrate a general and robust method of creating polymeric hemispherical shells via interfacial free-radical polymerization on the Janus droplets. The polymeric hemispherical shells were characterized by optical and fluorescence microscopy, scanning electron microscopy, and confocal laser scanning microscopy. By comparing phase diagrams of a regular Janus droplet and a Janus droplet with the hemispherical shell, we show that the formation of the hemispherical shell nearly doubles the range of the Janus morphology and maintains the Janus morphology upon a certain degree of external perturbation (e.g., adding hydrocarbon–water or fluorocarbon–water surfactants). We attribute the increased stability of the Janus droplets to (1) the surfactant nature of polymeric shell formed and (2) increase in interfacial tension between hydrocarbon and fluorocarbon due to polymer shell formation. This finding opens the door of utilizing these stabilized Janus droplets in a demanding environment.
Exciton migration to emissive defects in π-conjugated polymers is a robust signal amplification strategy for optoelectronic sensors. Herein we report end-capped conjugated polymers that show two distinct emissions as a function of interpolymer distances at the air–water and hydrocarbon–water interfaces. Amphiphilic poly(phenylene ethynylene)s (PPEs) end-capped with perylene monoimides display two distinct emission colors (cyan from PPE and red from perylene), the relative intensity of which depends on the surface pressure applied on the Langmuir monolayers. This behavior produces a ratiometric interfacial pressure indicator. Relative quantum yields are maintained at the different surface pressures and hence display no sign of self-quenching of the excitons in an aggregated state. These polymers can be organized at the micelle–water interface in lytropic liquid crystals, thereby paving the way for potential applications of end-capped amphiphilic conjugated polymers in biosensors and bioimaging.
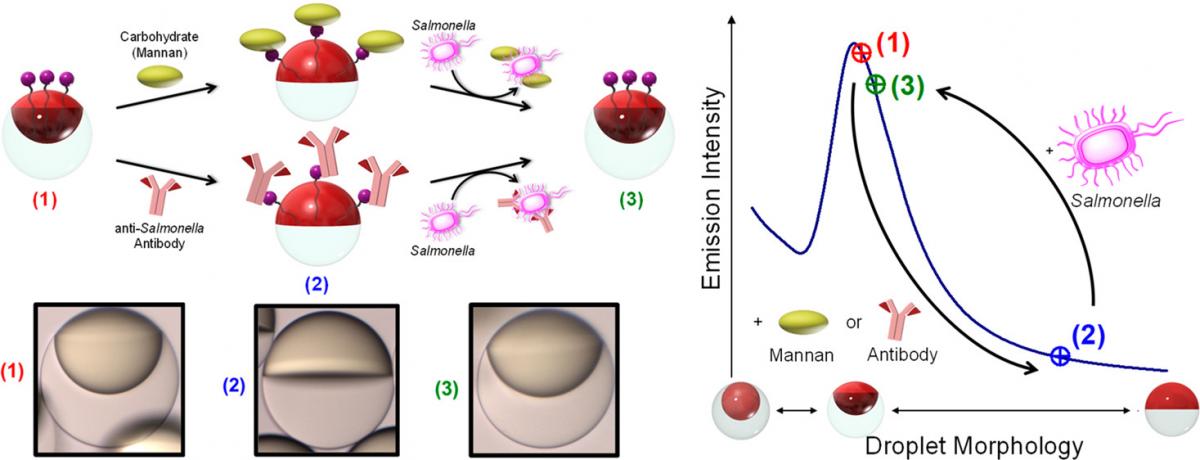
Janus emulsion assays that rely on carbohydrate–lectin binding for the detection of Escherichia coli bacteria are described. Surfactants containing mannose are self-assembled at the surface of Janus droplets to produce particles with lectin binding sites. Janus droplets orient in a vertical direction as a result of the difference in densities between the hydrocarbon and fluorocarbon solvents. Binding of lectin to mannose(s) causes agglutination and a tilted geometry. The distinct optical difference between naturally aligned and agglutinated Janus droplets produces signals that can be detected quantitatively. The Janus emulsion assay sensitively and selectively binds to E. coli at 104 cfu/mL and can be easily prepared with long-time stability. It provides the basis for the development of inexpensive portable devices for fast, on-site pathogen detection.
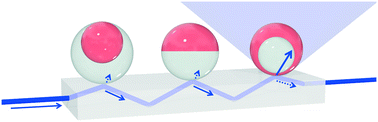
Micro-scale optical components play a crucial role in imaging and display technology, biosensing, beam shaping, optical switching, wavefront-analysis, and device miniaturization. Herein, we demonstrate liquid compound micro-lenses with dynamically tunable focal lengths. We employ bi-phase emulsion droplets fabricated from immiscible hydrocarbon and fluorocarbon liquids to form responsive micro-lenses that can be reconfigured to focus or scatter light, form real or virtual images, and display variable focal lengths. Experimental demonstrations of dynamic refractive control are complemented by theoretical analysis and wave-optical modelling. Additionally, we provide evidence of the micro-lenses’ functionality for two potential applications—integral micro-scale imaging devices and light field display technology—thereby demonstrating both the fundamental characteristics and the promising opportunities for fluid-based dynamic refractive micro-scale compound lenses.
Emissive molecules comprising a donor and an acceptor bridged by 9,9-dimethylxanthene, were studied (XPT, XCT, and XtBuCT). The structures position the donor and acceptor with cofacial alignment at distances of 3.3–3.5 Å wherein efficient spatial charge transfer can occur. The quantum yields were enhanced by excluding molecular oxygen and thermally activated delayed fluorescence with lifetimes on the order of microseconds was observed. Although the molecules displayed low quantum yields in solution, higher quantum yields were observed in the solid state. Crystal structures revealed π–π intramolecular interactions between a donor and an acceptor, however, the dominant intermolecular interactions were C—H···π, which likely restrict the molecular dynamics to create aggregation-induced enhanced emission. Organic light emitting devices using XPT and XtBuCT as dopants displayed electroluminescence external quantum efficiencies as high as 10%.
2016
A redox activated vase-to-kite conformational change is reported for a new resorcinarene-based cavitand appended with four quinoxaline-fused thianthrene units. In its neutral state, the thianthrene-containing cavitand was shown by 1H NMR to adopt a closed vase conformation. Upon oxidation the electrostatic repulsion among the thianthrene radical cations promotes a kite conformation in the thianthrene-containing cavitand. The addition of acid produced a shoulder feature below 300 nm in the cavitand’s UV-Vis spectrum that we have assigned to the vase-to-kite conformation change. UV-Vis spectroelectrochemical studies of the cavitand revealed a development of a similar shoulder peak consistent with the oxidation-induced vase-to-kite conformation change. To support that the shoulder peak is diagnostic for a vase-to-kite conformation change, a model molecule constituting a single quinoxaline wall of the cavitand was synthesized and studied. As expected UV-Vis spectroelectrochemical studies of the cavitand arm did not display a shoulder peak below 300 nm. The oxidation-induced vase-to-kite conformation is further confirmed by the distinctive upfield shift in 1H chemical shift of the methine signal.
A chemiresistive detector for carbon monoxide was created from single-walled carbon nanotubes (SWCNTs) by noncovalent modification with diiodo(η5:η1-1-[2-(N,N-dimethylamino)ethyl]-2,3,4,5-tetramethylcyclopentadienyl)-cobalt(III) ([Cp∧CoI2]), an organocobalt complex with an intramolecular amino ligand coordinated to the metal center that is displaced upon CO binding. The unbound amino group can subsequently be transduced chemiresistively by the SWCNT network. The resulting device was shown to have a ppm-level limit of detection and unprecedented selectivity for CO gas among CNT-based chemiresistors. This work, the first molecular-level mechanistic elucidation for a CNT-based chemiresistive detector for CO, demonstrates the efficacy of using an analyte’s reactivity to produce another chemical moiety that is readily transduced as a strategy for the rational design of chemiresistive CNT-based detectors.
Covalent functionalization significantly enhances the utility of carbon nanomaterials for many applications. Herein, we report an efficient method for the covalent functionalization of carbon nanotubes (CNTs) and graphite. This reaction involves the reduction of carbon nanomaterials with sodium naphthalide, followed by the addition of diaryliodonium salts. CNTs, including single-walled, double-walled, and multi-walled variants (SWCNTs, DWCNTs, and MWCNTs, respectively), as well as graphite, can be efficiently functionalized with substituted arene and heteroarene iodonium salts. The preferential transfer of phenyl groups containing electron-withdrawing groups was demonstrated by reactions with unsymmetrical iodonium salts. The lower reactivity of iodonium salts, relative to the more commonly used diazonium ions, presents opportunities for greater diversity in the selective functionalization of carbon nanomaterials.
Interactions between π-conjugated polymers are known to create ground-state aggregates, excimers, and exciplexes. With few exceptions, these species exhibit decreased fluorescence quantum yields relative to the isolated polymers in liquid or solid solutions. Herein, we report a method to assemble emissive conjugated polymer excimers and demonstrate their applicability in the detection of selected solvent vapors. Specifically, poly(phenylene ethynylene)s (PPEs) with amphiphilic side chains are organized in a Langmuir monolayer at the air–water interface. Compression of the monolayer results in the reversible conversion from a face-on organization of the π-system relative to the water to what appears to be an incline-stack conformation. The incline-stack organization creates a bright yellow emissive excimeric state with increases of 28% in relative fluorescence quantum yields to the face-on monolayer conformation. Multilayers can be transferred onto the glass substrate via a Langmuir–Blodgett method with preservation of the excimer emission. These films are metastable and the fluorescence reverts to a cyan color similar to the spectra obtained in solution and spin-cast films after exposure to selected solvent vapors. This behavior has practical utility as a fluorescence-based indicator for selected volatile organic compounds.
Iptycenes are intriguing compounds receiving considerable attention as a result of their rigid noncompliant three-dimensional architecture. The preparation of larger iptycenes is often problematic, as a result of their limited solubility and synthetic procedures involving multiple Diels–Alder reactions under harsh extended reaction conditions. We report a mechanochemical synthesis of structurally well-defined iptycenes through an iterative reaction sequence, wherein Diels–Alder reactions and a subsequent aromatization afford higher order iptycenes. We further report that double Diels–Alder reactions under solvent-free condition provide facile access to highly functionalized iptycenes with molecular weights over 2000 Da. Quartz crystal microbalance measurements reveal that these materials efficiently absorb the aromatic hydrocarbons benzene and toluene.
A platform for chemiresistive gas detectors based upon single-walled carbon nanotube (SWCNT) dispersions stabilized by poly(4-vinylpyridine) (P4VP) covalently immobilized onto a glass substrate was developed. To fabricate these devices, a glass substrate with gold electrodes is treated with 3-bromopropyltrichlorosilane. The resulting alkyl bromide coating presents groups that can react with the P4VP to covalently bond (anchor) the polymer–SWCNT composite to the substrate. Residual pyridyl groups in P4VP not consumed in this quaternization reaction are available to coordinate metal nanoparticles or ions chosen to confer selectivity and sensitivity to target gas analytes. Generation of P4VP coordinated to silver nanoparticles produces an enhanced response to ammonia gas. The incorporation of soft Lewis acidic Pd2+ cations by binding PdCl2 to P4VP yields a selective and highly sensitive device that changes resistance upon exposure to vapors of thioethers. The latter materials have utility for odorized fuel leak detection, microbial activity, and breath diagnostics. A third demonstration makes use of permanganate incorporation to produce devices with large responses to vapors of volatile organic compounds that are susceptible to oxidation.
Chemical sensors offer opportunities for improving personal security, safety, and health. To enable broad adoption of chemical sensors requires performance and cost advantages that are best realized from innovations in the design of the sensing (transduction) materials. Ideal materials are sensitive and selective to specific chemicals or chemical classes and provide a signal that is readily interfaced with portable electronic devices. Herein we report that wrapping single walled carbon nanotubes with metallo-supramolecular polymers creates sensory devices with a dosimetric (time- and concentration-integrated) increase in electrical conductivity that is triggered by electrophilic chemical substances such as diethylchlorophosphate, a nerve agent simulant. The mechanism of this process involves the disassembly of the supramolecular polymer, and we demonstrate its utility in a wireless inductively powered sensing system based on near-field communication technology. Specifically, the dosimeters can be powered and read wirelessly with conventional smartphones to create sensors with ultratrace detection limits.
Human exposure to hazardous chemicals can have adverse short- and long-term health effects. In this Communication, we have developed a single-use wearable hazard badge that dosimetrically detects diethylchlorophosphate (DCP), a model organophosphorous cholinesterase inhibitor simulant. Improved chemically actuated resonant devices (CARDs) are fabricated in a single step and unambiguously relate changes in chemiresistance to a wireless readout. To provide selective and readily manufacturable sensor elements for this platform, we developed an ionic-liquid-mediated single walled carbon nanotube based chemidosimetric scheme with DCP limits of detection of 28 ppb. As a practical demonstration, an 8 h workday time weighted average equivalent exposure of 10 ppb DCP effects an irreversible change in smartphone readout.
Strategies for the facile fabrication of nanoscale materials and devices represent an increasingly important challenge for chemists. Here{,} we report a simple{,} one-pot procedure for the formation of perfluorocarbon emulsions with defined functionalization. The fluorous core allows for small molecules containing a fluorous tail to be stabilized inside the emulsions. The emulsions can be formed using a variety of hydrophilic polymers resulting in an array of sizes (90 nm to >1 micron) and surface charges (-95 mV to 65 mV) of fluid particles. The surface of the emulsions can be further functionalized{,} covalently or non-covalently{,} through in situ or post-emulsion modification. The total preparation time is 30 minutes or less from commercially available reagents without specialized equipment. We envision these emulsions to be applicable to both biological and materials systems.
A highly efficient thin-film luminescent solar concentrator (LSC) utilizing two π-conjugated polymers as antennae for small amounts of the valued perylene bisimide Lumogen F Red 305 is presented. The LSC exhibits high photoluminescence quantum yield, low reabsorption, and relatively low refractive indices for waveguide matching. A Monte Carlo simulation predicts the LSC to possess exceptionally high optical efficiencies on large scales.
2015
Embodiments described herein provide functionalized carbon nanostructures for use in various devices, including photovoltaic devices (e.g., solar cells). In some cases, the carbon nanostructures are fullerenes substituted with one or more isobenzofulvene species and/or indane species. Devices including such materials may exhibit increased efficiency, increased open circuit potential, high electron/hole mobility, and/or low elec. resistance. [on SciFinder(R)]
Applications of porous metal-org. frameworks (MOFs) in electronic devices are rare, owing in large part to a lack of MOFs that display elec. cond. Here, we describe the use of conductive two-dimensional (2D) MOFs as a new class of materials for chemiresistive sensing of volatile org. compds. (VOCs). We demonstrate that a family of structurally analogous 2D MOFs can be used to construct a cross-reactive sensor array that allows for clear discrimination between different categories of VOCs. Exptl. data show that multiple sensing mechanisms are operative with high degrees of orthogonality, establishing that the 2D MOFs used here are mechanistically unique and offer advantages relative to other known chemiresistor materials.
A chemosensory system is reported that operates without the need for sepn. techniques and is capable of identifying anions and structurally similar bioactive mols. In this strategy, the coordination of analytes to a metal complex with an open binding cleft generates \"static structures\" on the NMR timescale. Unique signals are created by strategically placing fluorine atoms in close proximity to bound analytes so that small structural differences induce distinct 19F NMR shifts that can be used to identify each analyte. The utility of this method is illustrated by quantifying caffeine levels in coffee, by identifying ingredients in tea and energy drinks, and by discriminating between multiple biogenic amines with remote structural differences six carbon atoms away from the binding site. We further demonstrate the simultaneous identification of multiple neutral and anionic species in a complex mixture
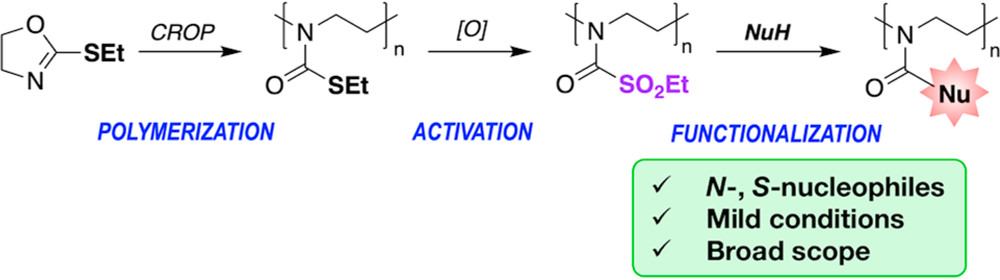
The threat of chem. warfare agents (CWAs) necessitates the development of functional materials that not only quickly detect the presence of CWAs but also actively protect against their toxicity. The authors have synthesized responsive units that exhibit colorimetric responses upon exposure to CWAs and incorporated them into a versatile detection platform based on copolymers prepd. by ring-opening metathesis polymn. (ROMP). The theor. detection limits for CWA simulants in soln. for these polymers are as low as 1 ppm. By incorporating hydrogel-promoting units as pendant chains, the authors are able to obtain polymers that instantly respond to CWA vapors and are easy to regenerate to the deactivated state by simple treatment with ammonium hydroxide vapor. The authors further demonstrate a collapse of the polymer gels in response to trifluoroacetic acid (TFA), a strong acid that produces a more fully ionized state as a result of its more caustic nature.
Architecture represents a promising yet underutilized control element in polymer design due to the challenging synthesis of compositionally varied branched copolymers. We report the one-pot synthesis of miktoarm branched polymers by ring-opening metathesis polymn. In this work, we graft to and from telechelic poly(3-hexylthiophene), which is end-capped by oxime click chem., using various norbornene monomers. The self-assembly of the resulting miktoarm H-shaped conjugated polymers is studied in soln. and in the solid state. A dual stimuli-responsive miktoarm polymer is prepd. that displays pH-switchable lower crit. soln. temp. and fluorescence.
Donor–acceptor triptycences, TPA-QNX(CN)2 and TPA-PRZ(CN)2, were synthesized and their emissive properties were studied. They exhibited a blue-green fluorescence with emission lifetimes on the order of a microsecond in cyclohexane at room temperature. The long lifetime emission is quenched by O2 and is attributed to thermally activated delayed florescence (TADF). Unimolecular TADF is made possible by the separation and weak coupling due to homoconjugation of the HOMO and LUMO on different arms of the three-dimensional donor–acceptor triptycene. Organic light emitting devices (OLEDs) were fabricated using TPA-QNX(CN)2 and TPA-PRZ(CN)2 as emitters which displayed electroluminescence with efficiencies as high as 9.4% EQE.
Abrupt switching behavior and near-zero leakage current of nanoelectromechanical (NEM) switches are advantageous properties through which NEMs can outperform conventional semiconductor electrical switches. To date, however, typical NEMs structures require high actuation voltages and can prematurely fail through permanent adhesion (defined as stiction) of device components. To overcome these challenges, in the present work we propose a NEM switch, termed a “squitch,” which is designed to electromechanically modulate the tunneling current through a nanometer-scale gap defined by an organic molecular film sandwiched between two electrodes. When voltage is applied across the electrodes, the generated electrostatic force compresses the sandwiched molecular layer, thereby reducing the tunneling gap and causing an exponential increase in the current through the device. The presence of the molecular layer avoids direct contact of the electrodes during the switching process. Furthermore, as the layer is compressed, the increasing surface adhesion forces are balanced by the elastic restoring force of the deformed molecules which can promote zero net stiction and recoverable switching. Through numerical analysis, we demonstrate the potential of optimizing squitch design to enable large on–off ratios beyond 6 orders of magnitude with operation in the sub-1 V regime and with nanoseconds switching times. Our preliminary experimental results based on metal–molecule–graphene devices suggest the feasibility of the proposed tunneling switching mechanism. With optimization of device design and material engineering, squitches can give rise to a broad range of low-power electronic applications.
Efficient methods for the preparation of functionalized metallated cavitands are described. Functional groups can be either introduced by an imidation of metal-oxo complexes or by a late-stage elaboration of the imido ligands. By using diversified iminophosphorane (PPh3=NR) reagents, π-conjugated pyrene, redox active ferrocene, and polymerizable norbornene moieties were successfully introduced. Furthermore, the iodo and alkynyl groups on the imido ligands are capable of undergoing efficient Sonogashira cross-coupling and copper-catalyzed azide alkyne cycloaddition reactions, thereby providing facile access to complex architectures containing metallated cavitands.
Chemiresistive detectors for amine vapors were made from single-walled carbon nanotubes by noncovalent modification with cobalt meso-arylporphyrin complexes. We show that through changes in the oxidation state of the metal, the electron-withdrawing character of the porphyrinato ligand, and the counteranion, the magnitude of the chemiresistive response to ammonia could be improved. The devices exhibited sub-ppm sensitivity and high selectivity toward amines as well as good stability to air, moisture, and time. The application of these chemiresistors in the detection of various biogenic amines (i.e. putrescine, cadaverine) and in the monitoring of spoilage in raw meat and fish samples (chicken, pork, salmon, cod) over several days was also demonstrated.
The utility of metal-organic frameworks (MOFs) as functional materials in electronic devices has been limited to date by a lack of MOFs that display high electrical conductivity. Here, we report the synthesis of a new electrically conductive 2D MOF, Cu3 (HITP)2 (HITP=2,3,6,7,10,11-hexaiminotriphenylene), which displays a bulk conductivity of 0.2 S cm(-1) (pellet, two-point-probe). Devices synthesized by simple drop casting of Cu3 (HITP)2 dispersions function as reversible chemiresistive sensors, capable of detecting sub-ppm levels of ammonia vapor. Comparison with the isostructural 2D MOF Ni3 (HITP)2 shows that the copper sites are critical for ammonia sensing, indicating that rational design/synthesis can be used to tune the functional properties of conductive MOFs.[on SciFinder (R)]
Embodiments described herein provide devices and methods for the detn. of analytes. The device typically includes an absorbent material that allows for an analyte sample to be concd. and analyzed simultaneously and within a short period of time (e.g., less than 10 s). Embodiments described herein can provide portable and easily operable devices for on-site, real time field monitoring with high sensitivity, selectivity, and fast response time. [on SciFinder(R)]
Emulsification is a powerful, well-known technique for mixing and dispersing immiscible components within a continuous liq. phase. Consequently, emulsions are central components of medicine, food and performance materials. Complex emulsions, including Janus droplets (i.e., droplets with faces of differing chemistries) and multiple emulsions, are of increasing importance in pharmaceuticals and medical diagnostics, in the fabrication of microparticles and capsules for food, in chem. sepns., in cosmetics, and in dynamic optics. Because complex emulsion properties and functions are related to the droplet geometry and compn., the development of rapid, simple fabrication approaches allowing precise control over the droplets' phys. and chem. characteristics is crit. Significant advances in the fabrication of complex emulsions have been made using a no. of procedures, ranging from large-scale, less precise techniques that give compositional heterogeneity using high-shear mixers and membranes, to small-vol. but more precise microfluidic methods. However, such approaches have yet to create droplet morphologies that can be controllably altered after emulsification. Reconfigurable complex liqs. potentially have great utility as dynamically tunable materials. Here we describe an approach to the one-step fabrication of three- and four-phase complex emulsions with highly controllable and reconfigurable morphologies. The fabrication makes use of the temp.-sensitive miscibility of hydrocarbon, silicone and fluorocarbon liqs., and is applied to both the microfluidic and the scalable batch prodn. of complex droplets. We demonstrate that droplet geometries can be alternated between encapsulated and Janus configurations by varying the interfacial tensions using hydrocarbon and fluorinated surfactants including stimuli-responsive and cleavable surfactants. This yields a generalizable strategy for the fabrication of multiphase emulsions with controllably reconfigurable morphologies and the potential to create a wide range of responsive materials. [on SciFinder(R)]
Embodiments described herein provide materials and methods for the absorption or filtration of various species and analytes. In some cases, the materials may be used to remove or reduce the amt. of a substance in vapor sample (e.g., cigarette smoke). [on SciFinder(R)]
Poly(3-hexylthiophene) (P3HT) is one of the most extensively investigated conjugated polymers and has been employed as the active material in many devices including field-effect transistors, org. photovoltaics and sensors. As a result, methods to further tune the properties of P3HT are desirable for specific applications. Herein, we report a facile postpolymn. modification strategy to functionalize the 4-position of com. available P3HT in two simple steps-bromination of the 4-position of P3HT (Br-P3HT) followed by lithium-bromine exchange and quenching with an electrophile. We achieved near quant. lithium-bromine exchange with Br-P3HT, which requires over 100 thienyl lithiates to be present on a single polymer chain. The lithiated-P3HT is readily combined with functional electrophiles, resulting in P3HT derivs. with ketones, secondary alcs., trimethylsilyl (TMS) group, fluorine, or an azide at the 4-position. We demonstrated that the azide-modified P3HT could undergo Cu-catalyzed or Cu-free click chem., significantly expanding the complexity of the structures that can be appended to P3HT using this method. [on SciFinder(R)]
The rapid detection and differentiation of chiral compds. is important to synthetic, medicinal, and biol. chem. Palladium complexes with chiral pincer ligands have utility in detg. the chirality of various amines. The binding of enantiomeric amines induces distinct 19F NMR shifts of the fluorine atoms appended on the ligand that defines a chiral environment around palladium. Further this method has the ability to evaluate the enantiomeric compn. and discriminate between enantiomers with chiral centers several carbons away from the binding site. The wide detection window provided by optimized chiral chemosensors allows the simultaneous identification of as many as 12 chiral amines. The extraordinary discriminating ability of this method is demonstrated by the resoln. of chiral aliph. amines that are difficult to sep. using chiral chromatog. [on SciFinder(R)]
We report the synthesis of dithienobenzotropone-based conjugated alternating copolymers by direct arylation polycondensation. Postpolymn. modification by hydride redn. yields cross-conjugated, reactive hydroxyl-contg. copolymers that undergo phosphorylation and ionization upon exposure to the chem. warfare agent mimic diethylchlorophosphate (DCP). The resulting conjugated, cationic copolymer is highly colored and facilitates the spectroscopic and colorimetric detection of DCP in both soln. and thin-film measurements. [on SciFinder(R)]
2014
Chemical sensing is of critical importance to human health, safety, and security, yet it is not broadly implemented because existing sensors often require trained personnel, expensive and bulky equipment, and have large power requirements. This study reports the development of a smartphone-based sensing strategy that employs chemiresponsive nanomaterials integrated into the circuitry of commercial near-field communication tags to achieve non-line-of-sight, portable, and inexpensive detection and discrimination of gas-phase chemicals (e.g., ammonia, hydrogen peroxide, cyclohexanone, and water) at part-per-thousand and part-per-million concentrations.
Detailed characterization of graphene oxide (GO) and its reduced forms continues to be a challenge. We have employed scanning tunneling microscopy (STM) to examine GO samples with varying degrees of deoxygenation via controlled chem. redn. Anal. of the roughness of the apparent height in STM topog. measurements, i.e. the äpparent roughness\", revealed a correlation between increasing deoxygenation and decreasing apparent roughness. This anal. can therefore be a useful supplement to the techniques currently available for the study of GO and related materials. The presence of a high elec. field underneath the STM tip can locally induce a reaction on the GO basal plane that leads to local deoxygenation, and the restoration of the sp2 hybridization of the carbons promotes increased planarity. These findings are in line with the apparent roughness values found for GO at varying levels of chem. redn. and illustrates the value of having a tool to gain structural/chem. insight on a local scale. This is the first example of employing an STM tip to locally reduce GO to reduced GO (rGO) and partially reduced GO (prGO) without locally destroying the graphene sample. Local manipulation on the nanoscale has utility for graphene nanoelectronics, and anal. employing the apparent roughness is an addnl. tool for the study of graphene oxide and related basal plane chem. [on SciFinder(R)]
We report a simple, rapid, and solvent-free methodol. for solid-state polymns. yielding poly(phenylene vinylenes) (PPVs) promoted by ball-milling. This solid-state Gilch polymn. method produces PPVs in as little as five minutes of milling. Detailed investigations of the parameter space governing the solid-state polymn., i.e., milling time, base strength, solid-state diln., milling frequency, and size of milling balls, revealed that polymn. by ball-milling is a rapid process achieving mol. no. av. wts. of up to 40 kDa in up to 70% yield. To explore the scope, a solid-state polymn. via the dithiocarbamate precursor route is explored. [on SciFinder(R)]
We report here the polymn. of several 7-isopropylidene-2,3-disubstituted norbornadienes, 7-oxa-2,3-dicarboalkoxynorbornadienes, and 11-oxa-benzonorbornadienes with a single tungsten oxo alkylidene catalyst, W(O)(CH-t-Bu)(OHMT)(Me2Pyr) (OHMT = 2,6-dimesitylphenoxide; Me2Pyr = 2,5-dimethylpyrrolide) to give cis, stereoregular polymers. The tacticities of the menthyl ester derivs. of two polymers were detd. for two types. For poly(7-isopropylidene-2,3-dicarbomenthoxynorbornadiene) the structure was shown to be cis, isotactic, while for poly(7-oxa-2,3-dicarbomenthoxynorbornadiene) the structure was shown to be cis, syndiotactic. A bis-trifluoromethyl-7-isopropylidene norbornadiene was not polymd. stereoregularly with W(O)(CHCMe2Ph)(Me2Pyr)(OHMT) alone, but a cis, stereoregular polymer was formed in the presence of 1 equiv of B(C6F5)3. [on SciFinder(R)]
We aimed to determine the incidence of Clostridium difficile infection (CDI), the molecular epidemiology of circulating C. difficile strains and risk factors for CDI among hospitalised children in the Auckland region. A cross-sectional study was undertaken of hospitalised children <15 years of age in two hospitals investigated for healthcare-associated diarrhoea between November 2011 and June 2012. Stool specimens were analysed for the presence of C. difficile using a two-step testing algorithm including polymerase chain reaction (PCR). C. difficile was cultured and PCR ribotyping performed. Demographic data, illness characteristics and risk factors were compared between children with and without CDI. Non-duplicate stool specimens were collected from 320 children with a median age of 1.2 years (range 3 days to 15 years). Forty-six patients (14 %) tested met the definition for CDI. The overall incidence of CDI was 2.0 per 10,000 bed days. The percentage of positive tests among neonates was only 2.6 %. PCR ribotyping showed a range of strains, with ribotype 014 being the most common. Significant risk factors for CDI were treatment with proton pump inhibitors [risk ratio (RR) 1.74, 95 % confidence interval (CI) 1.09-5.59; p = 0.002], presence of underlying malignancy (RR 2.71, 95 % CI 1.65-4.62; p = 0.001), receiving chemotherapy (RR 2.70, 95 % CI 1.41-4.83; p = 0.003) and exposure to antibiotics (RR 1.17, 95 % CI 0.99-1.17; p = 0.03). C. difficile is an important cause of healthcare-associated diarrhoea in this paediatric population. The notion that neonatal populations will always have high rates of colonisation with C. difficile may not be correct. Several risk factors associated with CDI among adults were also found to be significant.[on SciFinder (R)]
\"Fluoro\" refers to both fluorescent and fluorinated compds. Despite the shared prefix, there are very few fluorescent mols. that are sol. in perfluorinated solvents. This paucity is surprising, given that optical microscopy is a ubiquitous technique throughout the phys. sciences and the orthogonality of fluorous materials is a commonly exploited strategy in synthetic chem., materials science, and chem. biol. We have addressed this shortage by synthesizing a panel of \"fluorofluorophores,\" fluorescent mols. contg. high wt. percent fluorine with optical properties spanning the visible spectrum. We demonstrate the utility of these fluorofluorophores by prepg. fluorescent perfluorocarbon nanoemulsions. [on SciFinder(R)]
We report magic angle spinning, dynamic nuclear polarization (DNP) experiments at magnetic fields of 9.4 T, 14.1 T, and 18.8 T using the narrow line polarizing agents 1,3-bisdiphenylene-2-phenylallyl (BDPA) dispersed in polystyrene, and sulfonated-BDPA (SA-BDPA) and trityl OX063 in glassy glycerol/water matrices. The (1)H DNP enhancement field profiles of the BDPA radicals exhibit a significant DNP Overhauser effect (OE) as well as a solid effect (SE) despite the fact that these samples are insulating solids. In contrast, trityl exhibits only a SE enhancement. Data suggest that the appearance of the OE is due to rather strong electron-nuclear hyperfine couplings present in BDPA and SA-BDPA, which are absent in trityl and perdeuterated BDPA (d21-BDPA). In addition, and in contrast to other DNP mechanisms such as the solid effect or cross effect, the experimental data suggest that the OE in non-conducting solids scales favorably with magnetic field, increasing in magnitude in going from 5 T, to 9.4 T, to 14.1 T, and to 18.8 T. Simulations using a model two spin system consisting of an electron hyperfine coupled to a (1)H reproduce the essential features of the field profiles and indicate that the OE in these samples originates from the zero and double quantum cross relaxation induced by fluctuating hyperfine interactions between the intramolecular delocalized unpaired electrons and their neighboring nuclei, and that the size of these hyperfine couplings is crucial to the magnitude of the enhancements. Microwave power dependent studies show that the OE saturates at considerably lower power levels than the solid effect in the same samples. Our results provide new insights into the mechanism of the Overhauser effect, and also provide a new approach to perform DNP experiments in chemical, biophysical, and physical systems at high magnetic fields.[on SciFinder (R)]
On page 492, the 12th author's name was incomplete; the cor. name is given and the online version has been cor. [on SciFinder(R)]
Exciton fission is a process that occurs in certain org. materials whereby 1 singlet exciton splits into 2 independent triplets. In photovoltaic devices these 2 triplet excitons can each generate an electron, producing quantum yields per photon of >100% and potentially enabling single-junction power efficiencies >40%. Fission dynamics were measured using ultrafast photoinduced absorption and present a 1st-principles expression that successfully reproduces the fission rate in materials with vastly different structures. Fission is nonadiabatic and Marcus-like in weakly interacting systems, becoming adiabatic and coupling-independent at larger interaction strengths. In neat films, fission yields near unity were demonstrated even when monomers are sepd. by >5 \AA. For efficient solar cells, fission must outcompete charge generation from the singlet exciton. This work lays the foundation for tailoring mol. properties like soly. and energy level alignment while maintaining the high fission yield required for photovoltaic applications. [on SciFinder(R)]
Embodiments relating to the synthesis and processing of graphene mols. are provided. In some cases, methods for the electrochem. expansion and/or functionalization of graphene mols. are provided. In some embodiments, one or more species may be intercalated between adjacent graphene sheets. [on SciFinder(R)]
The development of new conjugated organic materials for dyes, sensors, imaging, and flexible light emitting diodes, field-effect transistors, and photovoltaics has largely relied upon assembling $π$-conjugated molecules and polymers from a limited number of building blocks. The use of the dithiolodithiole heterocycle as a conjugated building block for organic materials is described. The resulting materials exhibit complimentary properties to widely used thiophene analogues, such as stronger donor characteristics, high crystallinity, and a decreased HOMO-LUMO gap. The dithiolodithiole (C4S4) motif is readily synthetically accessible using catalytic processes, and both the molecular and bulk properties of materials based on this building block can be tuned by judicious choice of substituents.
\"Fluoro\" refers to both fluorescent and fluorinated compds. Despite the shared prefix, there are very few fluorescent mols. that are sol. in perfluorinated solvents. This paucity is surprising, given that optical microscopy is a ubiquitous technique throughout the phys. sciences and the orthogonality of fluorous materials is a commonly exploited strategy in synthetic chem., materials science, and chem. biol. We have addressed this shortage by synthesizing a panel of \"fluorofluorophores,\" fluorescent mols. contg. high wt. percent fluorine with optical properties spanning the visible spectrum. We demonstrate the utility of these fluorofluorophores by prepg. fluorescent perfluorocarbon nanoemulsions. [on SciFinder(R)]
A detector can detect an analyte including a carbon-carbon multiple bond moiety and capable of undergoing Diels-Alder reaction with a heteroarom. compd. having an extrudable group. The detector can detect, differentiate, and quantify ethylene. The detector can be a color based detector, a fluorescence based detector, or a resistivity based detector. [on SciFinder(R)]
Embodiments described herein provide functionalized carbon nanostructures for use in various devices, including photovoltaic devices (e.g., solar cells). In some embodiments, carbon nanostructures substituted with at least one cyclobutyl and/or cyclobutenyl group are provided. Devices including such materials may exhibit increased efficiency, increased open circuit potential, high electron/hole mobility, and/or low elec. resistance. [on SciFinder(R)]
Embodiments relating to the synthesis and processing of graphene mols. are provided. In some cases, methods for the electrochem. expansion and/or functionalization of graphene mols. are provided. In some embodiments, one or more species may be intercalated between adjacent graphene sheets. [on SciFinder(R)]
Improved methods for quickly identifying neutral org. compds. and differentiation of analytes with similar chem. structures are widely needed. We report a new approach to effectively \"fingerprint\" neutral org. mols. by using 19F NMR and mol. containers. The encapsulation of analytes induces characteristic up- or downfield shifts of 19F resonances that can be used as multidimensional parameters to fingerprint each analyte. The strategy can be achieved either with an array of fluorinated receptors or by incorporating multiple nonequivalent fluorine atoms in a single receptor. Spatial proximity of the analyte to the 19F is important to induce the most pronounced NMR shifts and is crucial in the differentiation of analytes with similar structures. This new scheme allows for the precise and simultaneous identification of multiple analytes in a complex mixt. [on SciFinder(R)]
We present a new templating approach that combines the templating properties of nanoporous networks with the dynamic properties and the lattice mismatch of domain boundaries. This templating approach allows for the inclusion of guests with different sizes without the need for a strict molecular design to tailor the nanoporous network. With this approach, nonperiodic patterns of functional molecules can be formed and studied. We show that domain boundaries in a trimesic acid network are preferred over pores within the network as adsorption sites for fullerenes by a factor of 100-200. Pristine fullerenes of different sizes and functionalized fullerenes were templated in this way.[on SciFinder (R)]
Embodiments described herein relate to compns. including iptycene-based structures and extended iptycene structures. The iptycene-based compd. comprises an iptycene core and at least one optionally substituted heterocyclyl or optionally substituted heteroaryl moiety rigidly bonded to the iptycene core, wherein the optionally substituted heterocyclyl or optionally substituted heteroaryl moiety defines at least a portion of the iptycene core. In some embodiments, the compns. may be useful in org. light-emitting diodes (OLEDs), org. photovoltaics, and other devices. [on SciFinder(R)]
We report magic angle spinning, dynamic nuclear polarization (DNP) expts. at magnetic fields of 9.4 T, 14.1 T, and 18.8 T using the narrow line polarizing agents 1,3-bisdiphenylene-2-phenylallyl (BDPA) dispersed in polystyrene, and sulfonated-BDPA (SA-BDPA) and trityl OX063 in glassy glycerol/water matrixes. The 1H DNP enhancement field profiles of the BDPA radicals exhibit a significant DNP Overhauser effect (OE) as well as a solid effect (SE) despite the fact that these samples are insulating solids. In contrast, trityl exhibits only a SE enhancement. Data suggest that the appearance of the OE is due to rather strong electron-nuclear hyperfine couplings present in BDPA and SA-BDPA, which are absent in trityl and perdeuterated BDPA (d21-BDPA). In addn., and in contrast to other DNP mechanisms such as the solid effect or cross effect, the exptl. data suggest that the OE in non-conducting solids scales favorably with magnetic field, increasing in magnitude in going from 5 T, to 9.4 T, to 14.1 T, and to 18.8 T. Simulations using a model two spin system consisting of an electron hyperfine coupled to a 1H reproduce the essential features of the field profiles and indicate that the OE in these samples originates from the zero and double quantum cross relaxation induced by fluctuating hyperfine interactions between the intramol. delocalized unpaired electrons and their neighboring nuclei, and that the size of these hyperfine couplings is crucial to the magnitude of the enhancements. Microwave power dependent studies show that the OE sats. at considerably lower power levels than the solid effect in the same samples. Our results provide new insights into the mechanism of the Overhauser effect, and also provide a new approach to perform DNP expts. in chem., biophys., and phys. systems at high magnetic fields. (c) 2014 American Institute of Physics. [on SciFinder(R)]
We report here the polymn. of several 7-isopropylidene-2,3-disubstituted norbornadienes, 7-oxa-2,3-dicarboalkoxynorbornadienes, and 11-oxa-benzonorbornadienes with a single tungsten oxo alkylidene catalyst, W(O)(CH-t-Bu)(OHMT)(Me2Pyr) (OHMT = 2,6-dimesitylphenoxide; Me2Pyr = 2,5-dimethylpyrrolide) to give cis, stereoregular polymers. The tacticities of the menthyl ester derivs. of two polymers were detd. for two types. For poly(7-isopropylidene-2,3-dicarbomenthoxynorbornadiene) the structure was shown to be cis, isotactic, while for poly(7-oxa-2,3-dicarbomenthoxynorbornadiene) the structure was shown to be cis, syndiotactic. A bis-trifluoromethyl-7-isopropylidene norbornadiene was not polymd. stereoregularly with W(O)(CHCMe2Ph)(Me2Pyr)(OHMT) alone, but a cis, stereoregular polymer was formed in the presence of 1 equiv of B(C6F5)3. [on SciFinder(R)]
Mech. abrasion is an extremely simple, rapid, and low-cost method for deposition of carbon-based materials onto a substrate. However, the method is limited in throughput, precision, and surface compatibility for drawing conductive pathways. Selective patterning of surfaces using laser-etching can facilitate substantial improvements to address these current limitations for the abrasive deposition of carbon-based materials. This study demonstrates the successful on-demand fabrication of fully-drawn chem. sensors on a wide variety of substrates (e.g., weighing paper, polymethyl methacrylate, silicon, and adhesive tape) using single-walled carbon nanotubes (SWCNTs) as sensing materials and graphite as electrodes. Mech. mixing of SWCNTs with solid or liq. selectors yields sensors that can detect and discriminate parts-per-million (ppm) quantities of various nitrogen-contg. vapors (pyridine, aniline, triethylamine). [on SciFinder(R)]
Polycyclic aromatic hydrocarbons (PAHs) and fully-conjugated ladder polymers are leading candidates for organics electronics, as their inherent conformational rigidity encourages electron delocalization. Many of these systems consist of fused benzenoid or heterocyclic aromatic rings. Less frequently, however, PAHs are reported with character that alternates between the aromaticity of benzene fragments and the antiaromaticity of a nonbenzenoid moiety. This chapter will focus on recent work published on the theory, synthesis, and properties of two such systems: [N]phenylenes containing 4$π$-electron cyclobutadienoid character, and diaryl[a,e]pentalenes containing 8$π$-electron pentalenoid character.[on SciFinder (R)]
We report a simple, rapid, and solvent-free methodol. for solid-state polymns. yielding poly(phenylene vinylenes) (PPVs) promoted by ball-milling. This solid-state Gilch polymn. method produces PPVs in as little as five minutes of milling. Detailed investigations of the parameter space governing the solid-state polymn., i.e., milling time, base strength, solid-state diln., milling frequency, and size of milling balls, revealed that polymn. by ball-milling is a rapid process achieving mol. no. av. wts. of up to 40 kDa in up to 70% yield. To explore the scope, a solid-state polymn. via the dithiocarbamate precursor route is explored. [on SciFinder(R)]
A series of conjugated cationic polymers, differentiated only by their accompanying counter-anions, was prepd. and characterized. The choice of counter-anion (CA) was found to drastically impact the soly. of the polymers and their optical properties in soln. and in the solid state. Fluorescent polymer thin films were found to be instantaneously quenched by volatile amines in the gas phase at low ppm concns., and a mini-array with CAs as variable elements was found to be able to differentiate amines with good fidelity. [on SciFinder(R)]
We present a new templating approach that combines the templating properties of nanoporous networks with the dynamic properties and the lattice mismatch of domain boundaries. This templating approach allows for the inclusion of guests with different sizes without the need for a strict molecular design to tailor the nanoporous network. With this approach, nonperiodic patterns of functional molecules can be formed and studied. We show that domain boundaries in a trimesic acid network are preferred over pores within the network as adsorption sites for fullerenes by a factor of 100-200. Pristine fullerenes of different sizes and functionalized fullerenes were templated in this way.
The synthesis of a covalently modified graphene oxide derivative with exceptional and tunable compressive strength is reported. Treatment of graphene oxide with triethyl phosphite in the presence of LiBr produces monolithic structures comprised of lithium phosphate oligomers tethered to graphene through covalent phosphonate linkages. Variation of the both phosphate content and associated cation produces materials of various compressive strengths and elasticity.
We illustrate the ability to place a water-insoluble biradical, bTbk, into a glycerol/water matrix with the assistance of a surfactant, sodium octyl sulfate (SOS). This surfactant approach enables a previously water insoluble biradical, bTbk, with favorable electron-electron dipolar coupling to be used for dynamic nuclear polarization (DNP) nuclear magnetic resonance (NMR) experiments in frozen, glassy, aqueous media. Nuclear Overhauser enhancement (NOE) and paramagnetic relaxation enhancement (PRE) experiments are conducted to determine the distribution of urea and several biradicals within the SOS macromolecular assembly. We also demonstrate that SOS assemblies are an effective approach by which mixed biradicals are created through an assembly process.
Cationic square planar Pt(II) complexes are reported with high degrees of intermolecular association. These complexes display thermotropic columnar liquid crystalline behavior in spite of having only a single side chain. Crystals undergo mechanochromic transformations that can be reversed with solvent.
We report our recent efforts directed at improving high-field dynamic nuclear polarization (DNP) expts. We investigated a series of thiourea nitroxide radicals and the assocd. DNP enhancements ranging from $ε$=25 to 82, which demonstrate the impact of mol. structure on performance. We directly polarized low-gamma nuclei, including 13C, 2H, and 17O, by the cross effect mechanism using trityl radicals as a polarization agent. We discuss a variety of sample prepn. techniques for DNP with emphasis on the benefits of methods that do not use a glass-forming cryoprotecting matrix. Lastly, we describe a corrugated waveguide for use in a 700 MHz/460 GHz DNP system that improves microwave delivery and increases enhancements up to 50 %. [on SciFinder(R)]
Development of a versatile method for incorporating conductive materials into textiles could enable advances in wearable electronics and smart textiles. One area of crit. importance is the detection of chems. in the environment for security and industrial process monitoring. Here, the fabrication of a flexible, sensor material based on functionalized multi-walled carbon nanotube (MWNT) films on a porous electrospun fiber mat for real-time detection of a nerve agent simulant is reported. The material is constructed by layer-by-layer (LbL) assembly of MWNTs with opposite charges, creating multilayer films of MWNTs without binder. The vacuum-assisted spray-LbL process enables conformal coatings of nanostructured MWNT films on individual electrospun fibers throughout the bulk of the mat with controlled loading and elec. cond. A thiourea-based receptor is covalently attached to the primary amine groups on the MWNT films to enhance the sensing response to di-Me methylphosphonate (DMMP), a simulant for sarin nerve agent. Chemiresistive sensors based on the engineered textiles display reversible responses and detection limits for DMMP as low as 10 ppb in the aq. phase and 5 ppm in the vapor phase. This fabrication technique provides a versatile and easily scalable strategy for incorporating conformal MWNT films into three-dimensional substrates for numerous applications. [on SciFinder(R)]
2013
The authors describe the first study of trinuclear gold(I) pyrazolates on the mol. level by time-dependent scanning tunneling microscopy (STM). On the graphite/1-octanoic acid interface, dodecyl-functionalized gold pyrazolates formed concn.-controlled morphologies. The authors found two types of monomeric packing and one dimeric type with two trinuclear gold pyrazolates next to each other on the surface. For an octadecyl-functionalized deriv., all studied concns. resulted in a dimeric morphol. However, different concns. led to different transient states during the layer evolution. At low concns., a transient monomeric state was present with the alkyl chains in a gauche-conformation that subsequently converted to a more optimized anti-conformation. At higher concns. a less stable \"line\" polymorph was obsd. The confinement of the mols. to the surface led to cooperative dynamics, in which two mols. in a dimer moved as if they were one particle. Furthermore, in a higher level of cooperativity, the rotation of one dimer appears to induce rotations in coupled neighboring dimers. [on SciFinder(R)]
Functionalized single-walled carbon nanotube (SWCNT)-based chemiresistors are reported for a highly robust and sensitive gas sensor to selectively detect cyclohexanone, a target analyte for explosive detection. The trifunctional selector has three important properties: it noncovalently functionalizes SWCNTs with cofacial $π$-$π$ interactions, it binds to cyclohexanone via hydrogen bond (mechanistic studies were studied), and it improves the overall robustness of SWCNT-based chemiresistors (e.g., humidity and heat). The authors' sensors produced reversible and reproducible responses in <30 s to 10 ppm of cyclohexanone and displayed an av. theor. limit of detection (LOD) of 5 ppm. [on SciFinder(R)]
PURPOSE: The goal was to identify molecular imaging probes that would enter the brain, selectively bind to Parkinson's disease (PD) pathology, and be detectable with one or more imaging modalities. PROCEDURE: A library of organic compounds was screened for the ability to bind hallmark pathology in human Parkinson's and Alzheimer's disease tissue, alpha-synuclein oligomers and inclusions in two cell culture models, and alpha-synuclein aggregates in cortical neurons of a transgenic mouse model. Finally, compounds were tested for blood-brain barrier permeability using intravital microscopy. RESULTS: Several lead compounds were identified that bound the human PD pathology, and some showed selectivity over Alzheimer's pathology. The cell culture models and transgenic mouse models that exhibit alpha-synuclein aggregation did not prove predictive for ligand binding. The compounds had favorable physicochemical properties, and several were brain permeable. CONCLUSIONS: Future experiments will focus on more extensive evaluation of the lead compounds as PET ligands for clinical imaging of PD pathology.[on SciFinder (R)]
During the three decades 1980-2010, magic angle spinning (MAS) NMR developed into the method of choice to examine many chemical, physical, and biological problems. In particular, a variety of dipolar recoupling methods to measure distances and torsion angles can now constrain molecular structures to high resolution. However, applications are often limited by the low sensitivity of the experiments, due in large part to the necessity of observing spectra of low-$\gamma$ nuclei such as the I = 1/2 species (13)C or (15)N. The difficulty is still greater when quadrupolar nuclei, such as (17)O or (27)Al, are involved. This problem has stimulated efforts to increase the sensitivity of MAS experiments. A particularly powerful approach is dynamic nuclear polarization (DNP) which takes advantage of the higher equilibrium polarization of electrons (which conventionally manifests in the great sensitivity advantage of EPR over NMR). In DNP, the sample is doped with a stable paramagnetic polarizing agent and irradiated with microwaves to transfer the high polarization in the electron spin reservoir to the nuclei of interest. The idea was first explored by Overhauser and Slichter in 1953. However, these experiments were carried out on static samples, at magnetic fields that are low by current standards. To be implemented in contemporary MAS NMR experiments, DNP requires microwave sources operating in the subterahertz regime, roughly 150-660 GHz, and cryogenic MAS probes. In addition, improvements were required in the polarizing agents, because the high concentrations of conventional radicals that are required to produce significant enhancements compromise spectral resolution. In the last two decades, scientific and technical advances have addressed these problems and brought DNP to the point where it is achieving wide applicability. These advances include the development of high frequency gyrotron microwave sources operating in the subterahertz frequency range. In addition, low temperature MAS probes were developed that permit in situ microwave irradiation of the samples. And, finally, biradical polarizing agents were developed that increased the efficiency of DNP experiments by factors of ∼4 at considerably lower paramagnet concentrations. Collectively, these developments have made it possible to apply DNP on a routine basis to a number of different scientific endeavors, most prominently in the biological and material sciences. This Account reviews these developments, including the primary mechanisms used to transfer polarization in high frequency DNP, and the current choice of microwave sources and biradical polarizing agents. In addition, we illustrate the utility of the technique with a description of applications to membrane and amyloid proteins that emphasizes the unique structural information that is available in these two cases.
A review. Thanks to their unique optical and electrochem. properties, conjugated polymers have attracted considerable attention over the last 2 decades and resulted in numerous technol. innovations. In particular, their implementation in sensing schemes and devices was widely studied and produced a multitude of sensory systems and transduction mechanisms. Conjugated polymers possess numerous attractive features that make them particularly suitable for a broad variety of sensing tasks. They display sensory signal amplification (compared to their small-mol. counterparts) and their structures can easily be tailored to adjust soly., absorption/emission wavelengths, energy offsets for excited state electron transfer, and/or for use in soln. or in the solid state. This versatility has made conjugated polymers a fluorescence sensory platform of choice in the recent years. In this review, the authors highlight a variety of conjugated polymer-based sensory mechanisms together with selected examples from the recent literature. [on SciFinder(R)]
We report the concise synthesis of a symmetrical monomer that provides a head-to-head pyridine building block for the preparation of cationic conjugated polymers. The obtained poly(pyridinium-phenylene) polymers display appealing properties such as high electron affinity, charge-transport upon n-doping, and optical response to electron-donating analytes. A simple assay for the optical detection of low micromolar amounts of a variety of analytes in aqueous solution was developed. In particular, caffeine could be measured at a 25 $μ$M detection limit. The reported polymers are also suitable for layer-by-layer film formation.
Chemiresistive sensor arrays for cyclohexanone and nitromethane are fabricated using single-walled carbon nanotubes (SWCNTs) that are covalently functionalized with urea, thiourea, and squaramide contg. selector units. Based on initial sensing results and 1H NMR binding studies, the most promising selectors are chosen and further optimized. These optimized selectors are attached to SWCNTs and simultaneously tested in a sensor array. The sensors show a very high level of reproducibility between measurements with the same sensor and across different sensors of the same type. Furthermore, the sensors show promising long-term stability, which renders them suitable for practical applications. [on SciFinder(R)]
Metathesis step-growth polymns. in ionic liqs. (ILs) was explored to take advantage of the high b.ps. of ILs, thereby permitting the use of low pressures at high temps. Optimization reactions found that high polymers form efficiently using small amts. of catalyst and short reaction times. For example, high mol. wt. main-chain triptycene polymers with high triptycene incorporation were synthesized. This new methodol. is applicable to various metathesis reactions that require removal of volatile byproducts as a driving force, including acyclic diene metathesis (ADMET). [on SciFinder(R)]
We present electron paramagnetic resonance experiments for which solid effect dynamic nuclear polarization transitions were observed indirectly via polarization loss on the electron. This use of indirect observation allows characterization of the dynamic nuclear polarization (DNP) process close to the electron. Frequency profiles of the electron-detected solid effect obtained using trityl radical showed intense saturation of the electron at the usual solid effect condition, which involves a single electron and nucleus. However, higher order solid effect transitions involving two, three, or four nuclei were also observed with surprising intensity, although these transitions did not lead to bulk nuclear polarization–suggesting that higher order transitions are important primarily in the transfer of polarization to nuclei nearby the electron. Similar results were obtained for the SA-BDPA radical where strong electron-nuclear couplings produced splittings in the spectrum of the indirectly observed solid effect conditions. Observation of high order solid effect transitions supports recent studies of the solid effect, and suggests that a multi-spin solid effect mechanism may play a major role in polarization transfer via DNP.
The hydroxyl functionalities in graphene oxide (GO), the vast majority that must be allylic alcs., have been subjected to Johnson-Claisen rearrangement conditions. Under these conditions, a [3, 3] sigmatropic rearrangement after reaction with tri-Et orthoacetate gives rise to an ester functional group, attached to the graphitic framework via a robust C-C bond. This variation of the Claisen rearrangement offers an unprecedented versatility of further functionalizations, while maintaining the desirable properties of unfunctionalized graphene. The resultant functional groups were found to withstand reductive treatments for the deoxygenation of graphene sheets and a resumption of electronic cond. is obsd. The ester groups are easily sapond. to carboxylic acids in situ with basic conditions, to give water-sol. graphene. The ester functionality can be further reacted as is, or the carboxylic acid can easily be converted to the more reactive acid chloride. Subsequent amide formation yields up to 1 amide in 15 graphene carbons and increases intergallery spacing up to 12.8 \AA, suggesting utility of this material in capacitors and in gas storage. Other functionalization schemes, which include the installation of terminal alkynes and dipolar cycloaddns., allow for the synthesis of a highly pos. charged, water-sol. graphene. The highly neg. and pos. charged graphenes (zeta potentials of -75 mV and +56 mV, resp.), are successfully used to build layer-by-layer (LBL) constructs. [on SciFinder(R)]
Fluorinated tungsten calix[4]arene imido complexes were synthesized and used as receptors to detect and differentiate neutral organic compounds. It was found that the binding of specific neutral organic molecules to the tungsten centers induces an upfield shift of the fluorine atom appended on the arylimido group, the extent of which is highly dependent on electronic and steric properties. We demonstrate that the specific bonding and size-selectivity of calix[4]arene tungsten-imido complex combined with (19)F NMR spectroscopy is a powerful new method for the analysis of complex mixtures.
We have developed highly fluorescent multiblock conjugated polymer nanoparticles for bioimaging and in vivo tumor targeting. We have shown that folate functionalized conjugated polymer nanoparticles exhibit preferential cell assocn. and uptake in vitro compared to non-functionalized nanoparticles. [on SciFinder(R)]
An octabenzo [12]cycloparaphenylene I (nanohoop) was prepd. in four steps as a potential precursor to a perylene-like cycloparaphenylene. Addn. of the lithium reagent derived from 1,4-dibromonaphthalene to 1,4-cyclohexanedione, O-methoxymethylation and isolation of the cis-dinaphthylcyclohexane, nickel-catalyzed macrocyclization, and acid- and microwave-mediated aromatization yielded I in 0.34% overall yield. The absorption and emission spectra of I and of its unaromatized precursor were obtained and compared. [on SciFinder(R)]
The accessible concn. of exfoliated and undamaged multi-walled carbon nanotubes (MWCNTs) in polymer nanocomposites is an essential issue to the future of these materials. In this work, we report two methodologies directed at obtaining elec. conducting poly(styrene-b-(ethylene-co-butylene)-b-styrene) (SEBS) nanocomposites with different MWCNT contents. The first depends on the time modulation of ultrasonication of toluene mixts., whereas the second relies on the use of alkyl-functionalized MWCNTs (f-MWCNTs). UV-vis spectroscopy investigations and thermogravimetric analyses allowed the quantification of exfoliated CNTs incorporated in the SEBS mixt. TEM micrographs denoted that a prolonged sonication time (40 min) induced an extensive MWCNTs degrdn. (av. length decreased of 40%), which affected the elec. cond. of the nanocomposites. The f-MWCNTs appeared to be more effective in prepg. SEBS nanocomposites due to the higher dispersion efficiency, negligible nanotube degrdn. and higher elec. cond.The temp. dependence of the resistance of the SEBS/MWCNT system was investigated in the range 20-60 °C to explore its potential for sensor development. [on SciFinder(R)]
Interfacial/in situ oxidative polymn. of polypyrrole in the presence of functionalized graphene sheets produces high-quality composites for supercapacitors, as analyzed by electrochem. impedance spectroscopy and cyclic voltammetry anal. The synergistic interaction induced by the growth of p-type polypyrrole on the surface of neg. charged carboxylate functionalized graphene sheets results in higher storage capacity than graphene-only or polymer-only films. The high cond. of p-doped polypyrrole and high surface area of graphene promote high charge accumulation in capacitors. The authors report the optimization of the relative concns. of carboxylate functionalized graphene in the polypyrrole matrix to maximize the compn.'s capacitance to 277.8 F/g. [on SciFinder(R)]
Chem. functionalized carbon nanotubes (CNTs) are promising materials for sensing of gases and volatile org. compds. However, the poor soly. of carbon nanotubes hinders their chem. functionalization and the subsequent integration of these materials into devices. This manuscript describes a solvent-free procedure for rapid prototyping of selective chemiresistors from CNTs and graphite on the surface of paper. This procedure enables fabrication of functional gas sensors from com. available starting materials in <15 min. The 1st step of this procedure involves the generation of solid composites of CNTs or graphite with small mol. selectors-designed to interact with specific classes of gaseous analytes-by solvent-free mech. mixing in a ball mill and subsequent compression. The 2nd step involves deposition of chemiresistive sensors by mech. abrasion of these solid composites onto the surface of paper. Parallel fabrication of multiple chemiresistors from diverse composites rapidly generates cross-reactive arrays capable of sensing and differentiating gases and volatile org. compds. at part-per-million and part-per-thousand concns. [on SciFinder(R)]
The synthesis of a long-lived, truxene-based radical that is highly delocalized and exhibits a narrow EPR absorption is reported. The radical is stable for multiple hours in a soln. exposed to air and remains for months in the solid state under inert gas. Characterization and properties are discussed. [on SciFinder(R)]
New tetraalkylcyclobutadiene-C60 adducts are developed via Diels-Alder cycloaddn. of C60 with in situ generated cyclobutadienes. The cofacial $π$-orbital interactions between the fullerene orbitals and the cyclobutene are shown to decrease the electron affinity and thereby increase the LUMO (LUMO) energy level of C60 significantly (ca. 100 and 300 meV for mono- and bisadducts, resp.). These variations in LUMO levels of fullerene can be used to generate higher open-circuit voltages (VOC) in bulk heterojunction polymer solar cells. The tetramethylcyclobutadiene-C60 monoadduct displays an open-circuit voltage (0.61 V) and a power conversion efficiency (2.49%) comparable to the widely used P3HT/PCBM (poly(3-hexylthiophene/([6,6]-phenyl-C61-butyric acid Me ester) composite (0.58 V and 2.57%, resp.). The role of the cofacial $π$-orbital interactions between C60 and the attached cyclobutene group was probed chem. by epoxidn. of the cyclobutene moiety and theor. through d. functional theory calcns. The electrochem., photophys., and thermal properties of the newly synthesized fullerene derivs. support the proposed effect of functionalization on electron affinities and photovoltaic performance. [on SciFinder(R)]
Three different types of epoxy-functionalized multi-walled carbon nanotubes (EpCNTs) were prepd. by multiple covalent functionalization methods. The EpCNTs were characterized by thermogravimetric anal. (TGA), IR spectroscopy (FTIR), and Raman spectroscopy to confirm covalent functionalization. The effect of the different chemistries on the adhesive properties was compared to a neat com. epoxy (Hexion formulation 4007) using functionalized and unfunctionalized multi-walled carbon nanotubes (MWCNT) at 0.5, 1, 2, 3, 5, and 10 wt%. It was found that an EpCNT at 1 wt% increased the lap shear strength, tested using the American Society for Testing and Materials std. test D1002, by 36% over the unfilled epoxy formulation and by 27% over a 1 wt% unmodified MWCNT control sample. SEM images revealed a fracture surface morphol. change with the incorporation of EpCNT and a deflection of the crack fronts at the site of embedded CNTs, as the mechanism accounting for increased adhesive strength. Rheol. studies showed non-linear viscosity and DSC cure studies showed an alteration of cure kinetics with increased CNT concn., and these effects were more pronounced for EpCNT. [on SciFinder(R)]
Several new triptycene-containing polyetherolefins were synthesized via acyclic diene metathesis (ADMET) polymerization. The well-established mechanism, high selectivity and specificity, mild reaction conditions, and well-defined end-groups make the ADMET polymerization a good choice for studying systematic variations in polymer structure. Two types of triptycene-based monomer with varying connectivities were used in the synthesis of homopolymers, block copolymers, and random copolymers. In this way, the influence of the triptycene architecture and concentration in the polymer backbone on the thermal behavior of the polymers was studied. Inclusion of increasing amounts of triptycene were found to increase the glass transition temperature, from 44 degrees C in polyoctenamer to 59 degrees C in one of the hydrogenated triptycene homopolymers (H-PT2). Varying the amounts and orientations of triptycene was found to increase the stiffness (H-PT1), toughness (PT11-b-PO1) and ductility (PT11-ran-PO3) of the polymer at room temperature. (c) 2013 Wiley Periodicals, Inc. J Polym Sci Part A: Polym Chem, 2013, 51, 1695-1706
Several solvent-free processing methods to disperse multiwalled carbon nanotubes (MWCNTs) in bisphenol F-based epoxy resin were investigated, including the use of a microfluidizer (MF), planetary shear mixer (PSM), ultrasonication (US) and combinations. The processed mixture was cured with diethyl toluene diamine. Three complimentary techniques were used to characterize the dispersion of the MWCNTs in cured composite samples: optical microscopy, micro Raman spectroscopy, and scanning electron microscopy (SEM). For sample MF + PSM, optical micrographs and Raman images showed reduced agglomeration and a homogeneous distribution of MWCNTs in the epoxy matrix. SEM analysis of fractured specimen after tensile testing revealed breakage of nanotubes along the fracture surface of the composite. A comparison of the MWCNT dispersion in the epoxy samples processed using different methods showed that a combination of MF and PSM processing yields a more homogeneous sample than the PSM or US + PSM processed samples. Mechanical testing of the composites showed about 15% improvement in the tensile strength of samples processed by the MF + PSM method over other methods. Thermogravimetric analysis (TGA) results showed a small decrease in the onset degradation temperature for poorly dispersed samples produced by PSM compared with the well-mixed samples (MF + PSM). These results strongly suggest that the MF + PSM processing method yield better-dispersed and stronger MWCNT/epoxy composites. (c) 2012 Wiley Periodicals, Inc. J Polym Sci Part B: Polym Phys, 2013
Photoalignment of nematic liquid crystals is demonstrated using a di-$π$-methane rearrangement of a designed polymer. The alignment mechanism makes use of the strong coupling of the liquid crystal directors to dibenzobarrelene groups. The large structural changes that accompany photoisomerization effectively passivate segments of the polymer, allowing the remaining dibenzobarrelene groups to dominate the director alignment. Photoisomerization requires triplet sensitization, and the polymer was designed to have a uniaxially fixed rigid structure and rapid triplet energy transfer from the proximate benzophenone units to the dibenzobarrelene groups. The isomerization was observed to be regiospecific, and thin films showed alignment.
We report direct (13)C dynamic nuclear polarization at 5 T under magic-angle spinning (MAS) at 82 K using a mixture of monoradicals with narrow EPR linewidths. We show the importance of optimizing both EPR linewidth and electron relaxation times by studying direct DNP of (13)C using SA-BDPA and trityl radical, and achieve (13)C enhancements above 600. This new approach may be best suited for dissolution DNP and for studies of (1)H depleted biological and other nonprotonated solids.
Dynamic nuclear polarization (DNP) of amorphous and crystalline ortho-terphenyl (OTP) in the absence of glass forming agents is presented in order to gauge the feasibility of applying DNP to pharmaceutical solid-state nuclear magnetic resonance experiments and to study the effect of intermolecular structure, or lack thereof, on the DNP enhancement. By way of (1)H-(13)C cross-polarization, we obtained a DNP enhancement ($ε$) of 58 for 95% deuterated OTP in the amorphous state using the biradical bis-TEMPO terephthalate (bTtereph) and $ε$ of 36 in the crystalline state. Measurements of the (1)H T1 and electron paramagnetic resonance experiments showed the crystallization process led to phase separation of the polarization agent, creating an inhomogeneous distribution of radicals within the sample. Consequently, the effective radical concentration was decreased in the bulk OTP phase, and long-range (1)H-(1)H spin diffusion was the main polarization propagation mechanism. Preliminary DNP experiments with the glass-forming anti-inflammation drug, indomethacin, showed promising results, and further studies are underway to prepare DNP samples using pharmaceutical techniques.
2012
Proteases are overexpressed in most cancers and proteolytic activity has been shown to be a viable marker for cancer imaging in vivo. Herein, we describe the synthesis of luminescence-quenched shell cross-linked nanoparticles as photonic nanoprobes for protease sensing. Protease sensing scheme is based on a \"turn-on\" mechanism where the protease cleaves peptide cross-linkers of the fluorescence-quenched shell cross-linked NP (OFF state) leading to a highly emissive non-cross linked NP (ON state). The cross-linked particles can be strained by exposure to a good solvent and proteolysis allows for particle expansion (swelling) and a recovery of the luminescence.
An all electrochemical route to functionalized graphene directly from a graphite electrode is described herein obviating the need for defect inducing oxidative or prolonged sonication treatments. Enhanced electrochemical expansion of graphite is achieved by sequential treatment, beginning with the established method of expansion by electrolysis in a Li(+) containing electrolyte, and then with the much larger tetra-n-butylammonium. The result is a hyperexpansion of the graphite basal planes. As a demonstration of the utility of this method, we successfully performed a subsequent in situ electrochemical diazonium functionalization of the hyperexpanded graphite basal planes to give functional graphene sheets. This potential controlled process is more effective than chemical processes and also provides a means of controlling the degree of functionalization. We have further demonstrated that the functionalized graphene could be converted to a pristine low defect form via laser ablation of the funtional groups. As a result, this method presents a potentially scalable approach for graphene circuit patterning.
The synthesis of air-stable, highly water-soluble organic radicals containing a 1,3-bis(diphenylene)-2-phenylallyl (BDPA) core is reported. A sulfonated derivative, SA-BDPA, retains the narrow electron paramagnetic resonance linewidth (<30 MHz at 5 T) of the parent BDPA in highly concentrated glycerol/water solutions (40 mM), which enables its use as polarizing agent for solid effect dynamic nuclear polarization (SE DNP). A sensitivity enhancement of 110 was obtained in high-field magic-angle-spinning (MAS) NMR experiments. The ease of synthesis and high maximum enhancements obtained with the BDPA-based radicals constitute a major advance over the trityl-type narrow-line polarization agents.
Single-walled carbon nanotubes (SWCNTs) have been functionalized with highly selective tetraphosphonate cavitand receptors. The binding of charged N-methylammonium species to the functionalized SWCNTs was analyzed by X-ray photoelectron spectroscopy and confirmed by (31)P MAS NMR spectroscopy. The cavitand-functionalized SWCNTs were shown to function as chemiresistive sensory materials for the detection of sarcosine and its ethyl ester hydrochloride in water with high selectivity at concentrations as low as 0.02 mM. Exposure to sarcosine and its derivative resulted in an increased conductance, in contrast to a decreased conductance response observed for potential interferents such as the structurally related glycine ethyl ester hydrochloride.
Lewis acids promote the polymerization of several 2-chloroalkylenedioxythiophenes, providing high-molecular-weight conjugated polymers. The proposed mechanism is a cationic chain-growth polymerization, as confirmed by end-capping reactions and a linear correlation of molecular weight with percent conversion. The \"living\" character of this process was used to prepare new block copolymers.
Synthesis of a new class of fully unsaturated ladder structures, phenylene-containing oligoacenes (POAs), using 3,4-bis(methylene)cyclobutene as a building block for sequential Diels-Alder reactions is described. The geometric effects of strain and energetic cost of antiaromaticity can be observed via the optical and electrochemical properties of the reported compounds. The resulting shape-persistant ladder structures contain neighboring chromophores that are partially electronically isolated from one another while still undergoing a reduction in the band gap of the material.
A new biradical polarizing agent, bTbtk-py, for dynamic nuclear polarization (DNP) experiments in aqueous media is reported. The synthesis is discussed in light of the requirements of the optimum, theoretical, biradical system. To date, the DNP NMR signal enhancement resulting from bTbtk-py is the largest of any biradical in the ideal glycerol/water solvent matrix, $ε$ = 230. EPR and X-ray crystallography are used to characterize the molecule and suggest approaches for further optimizing the biradical distance and relative orientation.
The synthesis and characterization of oxidized bis-thioketal-trispiro dinitroxide biradicals that orient the nitroxides in a rigid, approximately orthogonal geometry are reported. The biradicals show better performance as polarizing agents in dynamic nuclear polarization (DNP) NMR experiments as compared to biradicals lacking the constrained geometry. In addition, the biradicals display improved solubility in aqueous media due to the presence of polar sulfoxides. The results suggest that the orientation of the radicals is not dramatically affected by the oxidation state of the sulfur atoms in the biradical, and we conclude that a biradical polarizing agent containing a mixture of oxidation states can be used for improved solubility without a loss in performance.
The potential use of conjugated polymers in device applications is often limited by their less than optimal physicochem. properties. This work describes an efficient protocol to end-cap conjugated polymers synthesized via palladium-catalyzed cross-coupling polymns. with norbornene groups. Specifically, the hydroarylation of norbornadiene is shown to be a high-yielding end-capping method. These strained bicyclic alkenyl end groups can be transformed into macroinitiators via ring-opening metathesis polymn. and can polymerize other strained monomers, such as norbornene, yielding elastomeric triblock copolymers. [on SciFinder(R)]
We report a versatile new synthetic route to poly(para-phenylene)s (PPPs) and related poly(para-arylene)s containing high degrees of substitution not readily available by other methods. Our method transforms highly soluble, alkyne-containing polymers into PPPs via high-yielding, transition metal-mediated {[}2+2+2] cyclizations.
A review. A perspective on selected recent advances in graphene functionalization is offered. The activation of graphite as a means to create exfoliated sheets is highlighted as an important approach to achieve dispersions of individual graphene sheets. [on SciFinder(R)]
The motivation of this study is to determine if small amounts of designer additives placed at the polymer-fullerene interface in bulk heterojunction (BHJ) solar cells can influence their performance. A series of AB-alternating side-chain-functionalized poly(thiophene) analogues, P1-6, are designed to selectively localize at the interface between regioregular poly(3-hexylthiophene) (rr-P3HT) and PC(n)BM (n = 61, 71). The side chains of every other repeat unit in P1-6 contain various terminal aromatic moieties. BHJ solar cells containing ternary mixtures of rr-P3HT, PC(n)BM, and varying weight ratios of additives P1-6 are fabricated and studied. At low loadings, the presence of P1-6 consistently increases the short circuit current and decreases the series resistance of the corresponding devices, leading to an increase in power conversion efficiency (PCE) compared to reference P3HT/PC(61)BM cells. Higher additive loadings (>5 wt %) lead to detrimental nanoscale phase separation within the active layer blend and produce solar cells with high series resistances and low overall PCEs. Small-perturbation transient open circuit voltage decay measurements reveal that, at 0.25 wt % incorporation, additives P1-6 increase charge carrier lifetimes in P3HT/PC(61)BM solar cells. Pentafluorophenoxy-containing polymer P6 is the most effective side-chain-functionalized additive and yields a 28% increase in PCE when incorporated into a 75 nm thick rr-P3HT/PC(61)BM BHJ at a 0.25 wt % loading. Moreover, devices with 220 nm thick BHJs containing 0.25 wt % P6 display PCE values of up to 5.3% (30% PCE increase over a control device lacking P6). We propose that additives P1-6 selectively localize at the interface between rr-P3HT and PC(n)BM phases and that aromatic moieties at side-chain termini introduce a dipole at the polymer-fullerene interface, which decreases the rate of bimolecular recombination and, therefore, improves charge collection across the active layer.
A thin-film transistor: An n-type polymer semiconductor, poly(2,3-bis(perfluorohexyl)thieno[3,4-b]pyrazine), was synthesized through a Pd-catalyzed polycondensation employing a perfluorinated multiphase solvent system. This is the first example of an n-type polymer semiconductor with exclusive solubility in fluorinated solvents. The fabrication of organic field effect transistors containing this new n-type polymer semiconductor is shown.
A highly efficient method to introduce planarity and rigidity into a conjugated polymer backbone using a catalytic main-chain alteration to convert alkynes of PPE into annulated alkenes in PPV was developed. Post-polymn. cyclization provided a rare O-substitution of vinyl units of PPV and endowed products with greater planarity and rigidity, resulting in red-shifts in absorbance and fluorescence and defined vibrational features. We hope to extend this cyclization strategy to establish higher order of planarity for other conjugated polymer classes. [on SciFinder(R)]
Mech. abrasion of compressed single-walled carbon nanotubes (SWCNTs) on the surface of paper produces sensors capable of detecting NH3 gas at sub-ppm concns. This method of fabrication is simple, inexpensive, and entirely solvent-free, and avoids difficulties arising from the inherent instability of many SWCNT dispersions. [on SciFinder(R)]
Ethylene is the smallest ripening hormone in plants. In their Communication (DOI: 10.1002/anie.201201042), T. M. Swager et al. show that from a simple mixture of single-walled carbon nanotubes and a copper(I) complex, chemoresistive devices can be prepared that are highly sensitive and selective to sub-ppm concentrations of ethylene. The utility of the sensor was demonstrated by following ripening stages in different fruits.
The cyclization and planarization of polycyclic aromatic hydrocarbons with concomitant oxygen substitution was achieved through acid catalyzed transetherification and oxygen-radical reactions. The triptycene scaffold enforces proximity of the alcohol and arene reacting partners and confers significant rigidity to the resulting $π$-system, expanding the tool set of iptycenes for materials applications.
2010
The syntheses of two azaperylene 9,10-dicarboximides are presented. 1-Aza- and 1,6-diazaperylene 9,10-dicarboximides contg. a 2,6-diisopropylphenyl substituent at the N-imide position were synthesized in two steps starting from naphthalene and isoquinoline derivs. [on SciFinder(R)]
The design and synthesis of a water-soluble 1,3-bis(diphenylene)-2-phenylallyl (BDPA) radical via the conjugate addition of a derivatized fluorene nucelophile is described. The compound is designed for use in dynamic nuclear polarization NMR. Its 9 GHz EPR spectrum in glycerol/water is reported.
We report the design and synthesis of annulated thiepins designed to undergo bent-to-planar transformation driven by aromatization under electrochemical control. Thiepins are conjugated seven-membered ring systems with a thioether in the macrocycle. We synthesized thermally stable thiepins that are electropolymerizable to give rise to thiepin-containing electroactive polymers. Extended thiepin systems undergo sulfur extrusion with oxidation, and this feature has utility in peroxide sensing.
It's just a phase: A highly fluorinated compd. with a rigid three-dimensional architecture was synthesized as a monomer for poly(p-phenyleneethynylene)s (PPEs). Fluorous biphase reactions were applied for the synthesis of a PPE that is sol. only in fluorous solvents. A fluorous soln. of the polymer could be processed into aq. emulsions that display high fluorescence quantum yields. [on SciFinder(R)]
The sensing scheme the authors have designed for the detection of ethylene is based on a fluorescence turn-on mechanism and mimics nature by using a copper(I) complex to bind to ethylene. The fluorescence of the conjugated polymer is partially quenched by the presence of copper(I) moieties that can coordinate to the polymer. Upon exposure to ethylene gas, the copper complexes bind to the ethylene mols. and no longer quench the polymer fluorescence. The advantage of this fluorescence turn-on over a turn-off mechanism is that it requires a specific binding event to the copper to create a new signal, whereas fluorescence quenching can occur in multiple ways. Furthermore, if a completely dark background (completely quenched) state can be achieved, even a weak turn-on signal can be readily measured and thereby can allow trace detection. [on SciFinder(R)]
A novel sensing scheme that is not based on scintillation or charge generation in semiconductors can be deployed for the detection of uncharged ionization radiation using small devices. Functional POSs were accessed by click chem. methods, and several strategies were successfully deployed to increase sensor sensitivity. Systematic improvements in sensitivity can be accomplished by rational design, and incorporation of the appropriate chem. components to achieve sensitivities in the 103 rad range. [on SciFinder(R)]
A biocompatible post-polymerization functionalization reaction takes advantage of a polymer's structural motif for the controllable attachment of biotin as a model biosensor that responds to streptavidin.
We describe the synthesis of a series of phthalocyanine (Pc)-perylenediimide (PDI)(8) öctad\" molecules, in which eight PDI moieties are attached to a Pc core through alkyl-chain linkers. There is clear spectroscopic evidence that these octads can exist as non-aggregated \"monomers\" or form aggregates along the Pc cores, depending on the type of Pc and the solvent medium. In the low dielectric constant solvents, into which the octads are soluble, photoexcitation of the PDI units leads to rapid energy transfer to the Pc centre, rather than a charge separation between moieties. In octad monomers, the Pc singlet excited-state decays within tens of ps, whereas the excitons are stabilised in the aggregated form of the molecules, typically with lifetimes in the order of 1-10 ns. By contrast, in an octad design in which pi-pi interactions are suppressed by the steric hindrance of a corona of incompatible glycol tails around the molecule, a more straightforward photophysical interaction of F\örster energy transfer between the PDI moieties and Pc core may be inferred. We consider these molecules as prototypical multichromophoric aggregates, giving delocalised states with considerable flexibility of design.
A semi-conjugated iptycene polymer contg. a special \"crankshaft\" backbone leading to superior soly. in a nematic LC medium was synthesized. The incorporation of this polymer in the LC leads to thermodn. stabilization of the nematic mesophase. This effect is attributed to organizational coupling of the LC mols. to the polymer chains. [on SciFinder(R)]
Photoluminescent energy transfer was investigated in conjugated polymer-fluorophore blended thin films. A pentiptycene-contg. poly(phenyleneethynylene) was used as the energy donor, and 13 fluorophores were used as energy acceptors. The efficiency of energy transfer was measured by monitoring both the quenching of the polymer emission and the enhancement of the fluorophore emission. Near-IR emitting squaraines and terrylenes were identified as excellent energy acceptors. These results, where a new fluorescent signal occurs in the near-IR region on a completely dark background, offer substantial possibilities for designing highly sensitive turn-on sensors. © 2010 Wiley Periodicals, Inc. J Polym Sci Part A: Polym Chem 48: 3382-3391, 2010. [on SciFinder(R)]
Despite many advances in carbon nanotube (CNT) research, several issues continue to plague the field with regard to the construction of well-defined hybrid CNT materials. Regiospecific covalent functionalization, nonspecific surface absorption, and carbon nanotube aggregation/bundling present major difficulties when working with these materials. In this communication, we circumvent these problems and report a new addressable hybrid material composed of single-walled carbon nanotubes terminally linked by oligonucleotides into a nanowire motif. We show that the oligonucleotide junctions are addressable and can be targeted by gold nanoparticles.
Thioether-containing poly(para-phenylene-ethynylene) (PPE) copolymers show a strong fluorescence turn-on response when exposed to oxidants in solution as a result of the selective conversion of thioether substituents into sulfoxides and sulfones. We propose that the increase in fluorescence quantum yield (Phi(F)) upon oxidation is the result of both an increase in the rate of fluorescence (k(F)), as a result of greater spatial overlap of the frontier molecular orbitals in the oxidized materials, and an increase in the fluorescence lifetime (tau(F)), due to a decrease in the rate of nonradiative decay. Contrary to established literature, the reported sulfoxides do not always act as fluorescence quenchers. The oxidation is accompanied by spectral changes in the absorption and emission of the polymers, which are dramatic when oxidation causes the copolymer to acquire a donor-acceptor interaction. The oxidized polymers have high fluorescence quantum yields in the solid state, with some having increased photostability. A turn-on fluorescence response to hydrogen peroxide in organic solvents in the presence of an oxidation catalyst indicates the potential of thioether-containing materials for oxidant sensing. The reported polymers show promise as new materials in applications where photostability is important, where tunability of emission across the visible spectrum is desired, and where efficient emission is an advantage.
Stereoselective Diels-Alder cycloadditions that probe substituent effects in aryl-aryl sandwich complexes were studied experimentally and theoretically. Computations on model systems demonstrate that the stereoselectivity in these reactions is mediated by differential pi-stacking interactions in competing transition states. This allows relative stacking free energies of substituted and unsubstituted sandwich complexes to be derived from measured product distributions. In contrast to gas-phase computations, dispersion effects do not appear to play a significant role in the substituent effects, in accord with previous experiments. The experimental pi-stacking free energies are shown to correlate well with Hammett sigma(m) constants (r = 0.96). These substituent constants primarily provide a measure of the inductive electron-donating and -withdrawing character of the substituents, not donation into or out of the benzene pi-system. The present experimental results are most readily explained using a recently proposed model of substituent effects in the benzene sandwich dimer in which the pi-system of the substituted benzene is relatively unimportant and substituent effects arise from direct through-space interactions. Specifically, these results are the first experiments to clearly show that OMe enhances these pi-stacking interactions, despite being a pi-electron donor. This is in conflict with popular models in which substituent effects in aryl-aryl interactions are modulated by polarization of the aryl pi-system.
An elastomeric polymer composite that can be disassembled at will into its individual components when exposed to mild bases is presented. The composite is formed of a poly(olefin sulfone) and a silicone bound together using \"click\" chem. The mech. properties of the composites can be varied depending on their formulation. Its base-triggered decompn. is advantageous from the point of view of composite recycling and controlled release of chems. [on SciFinder(R)]
We report the synthesis of annulated azepines, conjugated seven-membered ring systems with nitrogen, designed to undergo an electrochem. controlled bent-to-planar transformation driven by aromatization. A Pd-catalyzed double amination strategy enabled us to synthesize annulated azepines, which are thermally and electrochem. stable even in highly oxidized states. Several methods of chem. and electrochem. polymn. yielded azepine-based materials that were demonstrated to retain their redox properties in the solid state under ambient conditions. Because of their redox stability and cond., these polymers could find utility in actuating materials research. [on SciFinder(R)]
Polycyclic arom. monomers based on substituted dibenz[a,h]anthracene frameworks have been prepd. in high yields. From these, several fluorescent stair-stepped conjugated polymers contg. fused arom. subunits have been synthesized via Sonogashira polycondensations and Glaser-type oxidative couplings. The new polymers were characterized by NMR spectroscopy, gel-permeation chromatog. (GPC), UV-vis, and fluorescence spectroscopy. [on SciFinder(R)]
The synthesis of precisely defined nanoscale hybrid materials remains a challenge at the frontier of chemistry and material science. In particular, the assembly of diverse high-aspect ratio one-dimensional materials such as gold nanorods and carbon nanotubes into functional systems is of ever increasing interest due to their electronic and sensing applications. To meet these challenges, methods for interfacing gold nanorods with carbon materials such as single-walled carbon nanotubes (SWCNTs) in a regio-controlled manner are needed. Herein, we report a method for the regiospecific synthesis of terminally linked gold nanorod-SWCNTs based on a nanotube surface protection strategy. The key to our approach is a SWCNT surface protection procedure allowing for selective functionalization of the SWCNT termini.
The synthesis of poly-1,3-bisdiphenylene-2-phenyl allyl (BDPA) radicals via a new anionic oligomerization strategy is reported. The material displays a reversible reduction from the orange-red radical to the blue carbanion in solution.
The efficient synthesis of a hydrophilic monomer bearing a three-dimensional noncompliant array of hydroxyl groups is described that prevents water-driven excimer features of hydrophobic poly(p-phenylene ethynylene) backbones. Sensitivity of the polymer to 3-nitrotyrosine is also discussed.
A series of 6,6-dicyanofulvene derivatives are synthesized starting from masked, dimeric, or monomeric cyclopentadienones. The reactivities of 6,6-dicyanofulvenes relative to their parent cyclopentadienones are discussed. 6,6-Dicyanofulvenes are capable of undergoing two consecutive, reversible, one-electron reductions and are presented as potential n-type small molecules.
2011
Low-cost, silicon-compatible on chip high-quality (Q) micro-ring visible lasers are prepd. by coating silica resonators with a combination of org. dyes - AlQ3, DCJTB, and Terrylene. The materials are selected to demonstrate cascaded energy transfer, which concs. the optical pump and overcomes the mismatch in the spectrally and spatially broad pump and the narrow modes of the high-finesse resonant cavity. The broadband pump excites a short wavelength dye in a low Q-factor cavity, there by avoiding the need to resonantly match the pump. Next, the low Q-factor cavity pumps a longer wavelength dye in what is effectively a higher Q-factor cavity, and the process can be repeated, until the desired Q-factor is met. All cavities are on the same resonator and differ only in the wavelength of their operation. Detuning of one cavity with respect to the other cannot occur, eliminating the instability normally assocd. with resonant pumping of high-Q lasers. [on SciFinder(R)]
The basal plane allylic alc. functionality of graphite oxide can be converted into N,N-dimethylamide groups through an Eschenmoser-Claisen sigmatropic rearrangement by using N,N-dimethylacetamide di-Me acetal. Subsequent sapon. of these groups affords the carboxylic acids, which, when deprotonated, electrostatically stabilize the graphene sheets in an aq. environment. [on SciFinder(R)]
The covalent, surface functionalization of graphene oxide with the malononitrile anion has been demonstrated. Once installed, these surface-bound \"molecular lynchpins\" can be chemically modified to increase the solubility of the graphene derivative in either organic or aqueous environments.
A review. From their unique elec. and mech. properties, carbon nanotubes (CNTs) have attracted great attention in recent years. A diverse array of methods was developed to modify CNTs and to assemble them into devices. From these innovations, many applications that include the use of CNTs were demonstrated. Transparent electrodes for org. light-emitting diodes (OLEDs), lithium-ion batteries, supercapacitors, and CNT-based electronic components such as field-effect transistors (FETs) were demonstrated. Also, CNTs were employed in catalysis and sensing as well as filters and mech. and biomedical applications. This review highlights illustrative examples from these areas to give an overview of applications of CNTs. [on SciFinder(R)]
Efficient synthesis of heteroleptic tris-cyclometalated Ir(III) complexes mer-Ir(C(/$\backslash$)N)(2)(trpy) (trpy = 2-(1H-[1,2,3]triazol-4-yl)pyridine) is achieved by using the Cu(I)-triazolide intermediates formed in \"click\" reactions as transmetalating reagents. Ligand preparation and cyclometalation of Ir(III) is accomplished in one pot. The robust nature of click chemistry provides opportunities to introduce different functional groups to the cyclometalated system, for example, alkyl, perfluoroalkyl, and aryl moieties. All of the meridional isomers show short-lived phosphorescence at room temperature, both in solution and in the solid state. DFT calculations indicates that the phosphorescence of mer-Ir(C(/$\backslash$)N)(2)(trpy) is attributed to the (3)MLCT and (3)LC mixed excited states, also supported by the broad spectral shape and hypsochromic shift upon media rigidification. The luminescence efficiency and excited state lifetimes of the cyclometalated complexes can be tuned by varying the substituents on the triazole ring, while the emission color is mainly determined by the phenylpyridine-based ligands. Moreover, the trpy ligand can acquire the N(/$\backslash$)N chelating mode under selective reaction conditions. mer-Ir(C(/$\backslash$)N)(2)(trpy) complexes isomerize into cationic [Ir(C(/$\backslash$)N)(2)(N(/$\backslash$)N\_trpy)](+) species instead of their fac isomers upon heating or UV radiation. This can be explained by the strong trans influence exerted by the phenyl groups. The weakened Ir-C(trpy) bonds are likely to be activated and protonated, leading to the switch of the trpy ligand to a thermodynamically more stable N(/$\backslash$)N chelating mode.
Highly water sol. sulfonate MWCNTs were synthesized and could be used to facilitate the electron transfer between Pd and Cu in a Wacker-type oxidn. in soln. [on SciFinder(R)]
Bispyridinium-phenylene-based conjugated donor-acceptor copolymers were synthesized by a Stille cross-coupling and cyclization sequence. These polyelectrolytes are freely sol. in org. solvents and display broad optical absorption bands that extend into the near-IR region. They show ambipolar redox properties with high electron affinities (LUMO levels) of 3.9-4.0 eV as well as high degrees of electroactivity. When reduced (n-doped) these materials display in situ conductivities as high as 180 S/cm. The high cond. is attributed to the planar structure that is enforced by the cyclic structures of the polymer. The electron affinities are compared to PCBM, a C60 based n-type material and hence may find utility in photovoltaic devices. [on SciFinder(R)]
A review. The ability of conjugated polymers to function as electronic materials is dependent on the efficient transport of excitons along the polymer chain. Generally, the photophysics of the chromophore monomer dictate the excited state behavior of the corresponding conjugated polymers. Different mol. structures are examd. to study the role of excited state lifetimes and mol. conformations on energy transfer. The incorporation of rigid, three-dimensional scaffolds, such as iptycenes and cyclophanes, can encourage an oblique packing of the chromophore units of a conjugated polymer, thus allowing the formation of electronically-coupled aggregates that retain high quantum yields of emission. Rigid iptycene scaffolds also act as excellent structural directors that encourage complete solvation of PPEs in a liq. crystal (LC) solvent. LC-PPE mixts. display both an enhanced conformational alignment of polymer chains and extended effective conjugation lengths relative to isotropic solns., which leads to enhanced energy transfer. Facile exciton migration in poly(p-phenylene ethynylene)s (PPEs) allows energy absorbed over large areas to be funneled into traps created by the binding of analytes, resulting in signal amplification in sensory devices. © 2011 Wiley Periodicals, Inc., J Polym Sci Part B: Polym Phys, 2011. [on SciFinder(R)]
A diverse array of multiwalled carbon nanotube (MWCNT) sensory materials have been synthesized and used to create sensors capable of identifying volatile organic compounds (VOCs) on the basis of their functional groups. Functionalized MWCNTs with a series of cross-sensitive recognition groups were successfully synthesized via zwitterionic and post-transformation synthetic procedures. The incorporated chemical functional groups on MWCNT surfaces introduced greatly increased sensitivity and selectivity to the targeted analytes. The distinct response pattern of each chemical was subjected to statistical treatments, which led to a clear separation and accurate identification of 100% of the VOCs. These results demonstrate that covalent functionalized MWCNT-based sensor arrays are a promising approach for low-cost, real time detection and identification of VOCs.
We detail our efforts toward the selective detection of cyclic ketones, e.g. cyclohexanone, a component of plasticized explosives. Thin films comprised of a conjugated polymer are used to amplify the emission of an emissive receptor via energy transfer. We propose that the energy transfer is dominated by an electron-exchange mechanism to an upper excited state of the fluorophore followed by relaxation and emission to account for the efficient energy transfer in the absence of appreciable spectral overlap. Exposure to cyclic ketones results in a ratiometric fluorescence response. The thin films show orthogonal responses when exposed to cyclic ketones versus acyclic ketones. We demonstrate that the exquisite selectivity is the result of a subtle balance between receptor design and the partition coefficient of molecules into the polymer matrix.
The ability to detect biological analytes in a rapid, sensitive, operationally simple, and cost-effective manner will impact human health and safety. Hybrid biocatalyzed-carbon nanotube (CNT) nanowire-based detection methods offer a highly sensitive and specific platform for the fabrication of simple and effective conductometric devices. Here, we report a conductivity-based DNA detection method utilizing carbon nanotube-DNA nanowire devices and oligonucleotide-functionalized enzyme probes. Key to our sensor design is a DNA-linked-CNT wire motif, which forms a network of interrupted carbon nanotube wires connecting two electrodes. Sensing occurs at the DNA junctions linking CNTs, followed by amplification using enzymatic metalization leading to a conductimetric response. The DNA analyte detection limit is 10 fM with the ability to discriminate single, double, and triple base pair mismatches. DNA-CNT nanowires and device sensing gaps were characterized by scanning electron microscopy (SEM) and confocal Raman microscopy, supporting the enhanced conductometric response resulting from nanowire metallization.
Conjugated cryst.-cryst. donor-acceptor-donor block copolymer semiconductors, with regioregular poly(3-hexylthiophene) as a donor (p-type) block and poly(pyridinium phenylene) as an acceptor (n-type) block within the backbone, were produced by sequential Grignard metathesis synthesis of poly(3-hexylthiophene), a Yamamoto-type cross-coupling polymn.-cyclization sequence. These conjugated block copolymers are sol. in org. solvents and display broad optical absorption bands extending close to the near-IR region. They show reversible ambipolar redox properties with high electron affinities of 3.8-4.0 eV as well as useful ionization potentials of 5.1 eV that are characteristic of the resp. blocks. Block copolymers from p- and n-type org. semiconductors are of interest for the formation of nanostructured bulk heterojunctions in photovoltaic devices. [on SciFinder(R)]
Rylene dyes functionalized with varying numbers of phenyl trifluorovinylether (TFVE) moieties were subjected to a thermal emulsion polymerization to yield shape-persistent, water-soluble chromophore nanoparticles. Perylene and terrylene diimide derivatives containing either two or four phenyl TFVE functional groups were synthesized and subjected to thermal emulsion polymerization in tetraglyme. Dynamic light scattering measurements indicated that particles with sizes ranging from 70 - 100 nm were obtained in tetraglyme, depending on monomer concentration. The photophysical properties of individual monomers were preserved in the nanoemulsions and emission colors could be tuned between yellow, orange, red, and deep red. The nanoparticles were found to retain their shape upon dissolution into water and the resulting water suspensions displayed moderate to high fluorescence quantum yield.
A series of sol., thermally stable arom. polyimides were synthesized using com. available five- and six-membered ring anhydrides and 2,6-diaminotriptycene derivs. All of these triptycene polyimides (TPIs) were sol. in common org. solvents despite their completely arom. structure due to the three-dimensional triptycene structure that prevents strong interchain interactions. Low soln. viscosities (0.07-0.47 dL/g) and versatile solubilities allow for easy soln. processing of these polymers. Nanoporosity in the solid state gives rise to high surface areas (up to 430 m2/g) and low refractive indexes (1.19-1.79 at 633 nm), which suggest very low dielec. consts. at optical frequencies. Polymer films were found to be amorphous. The decompn. temp. (Td) for all of the polymers is above 500°, and no glass transition temps. can be found below 450° by differential scanning calorimetry (DSC), indicating excellent prospects for high-temp. applications. This combination of properties makes these polymers candidates for spin-on dielec. materials. [on SciFinder(R)]
3-R-C60-Fullereno[6,6]isoxazolines (R = 2-pyridinyl, 2-MeOC6H4, 6-(2-MeOC6H4)C5H3N, 2,2'-bipyridin-6-yl) were prepd. by cycloaddn. of C60 with nitrile oxides RC≡N-O, generated in situ by dehydrohalogenation of hydroxyimidoyl chlorides RCX:NOH. This reaction provides access to an array of fullerene-fused heterocycles bearing covalently linked chelate moieties. The improved chelating property of the newly synthesized isoxazoline[60]fullerene adducts toward transition metals allows the syntheses of octahedral and square-planar organometallic compds. of rhenium, iridium, and platinum. This new approach has great potential as a general route to other novel derivs. contg. catalytically active transition metals. The redox properties of 3-(2-pyridinyl)fullereno[d]isoxazoline (2a) and its rhenium chlorotricarbonyl complexes were studied by cyclic voltammetry. [on SciFinder(R)]
The syntheses of several derivs. of 3,4-bis(benzylidene)cyclobutene I-V are reported. Previously unknown 1,2-dibromo-3,4-bis(benzylidene)cyclobuteneIII-V were obtained through in situ generation of 1,6-diphenyl-3,4-dibromo-1,2,4,5-tetraene followed by electrocyclic ring closure. Ensuing redn. and metal-catalyzed cross-coupling provided addnl. derivs. The effects of ring strain on the geometry and electronics of these derivs. were examd. by X-ray crystallog. and 1H NMR spectroscopy, resp. [on SciFinder(R)]
The nitramine-containing explosive RDX and the nitroester-containing explosive PETN are shown to be susceptible to photofragmentation upon exposure to sunlight. Model compounds containing nitroester and nitramine moieties are also shown to fragment upon exposure to UV irradiation. The products of this photofragmentation are reactive, electrophilic NO(x) species, such as nitrous and nitric acid, nitric oxide, and nitrogen dioxide. N,N-Dimethylaniline is capable of being nitrated by the reactive, electrophilic NO(x) photofragmentation products of RDX and PETN. A series of 9,9-disubstituted 9,10-dihydroacridines (DHAs) are synthesized from either N-phenylanthranilic acid methyl ester or a diphenylamine derivative and are similarly shown to be rapidly nitrated by the photofragmentation products of RDX and PETN. A new (turn-on) emission signal at 550 nm is observed upon nitration of DHAs due to the generation of fluorescent donor-acceptor chromophores. Using fluorescence spectroscopy, the presence of ca. 1.2 ng of RDX and 320 pg of PETN can be detected by DHA indicators in the solid state upon exposure to sunlight. The nitration of aromatic amines by the photofragmentation products of RDX and PETN is presented as a unique, highly selective detection mechanism for nitroester- and nitramine-containing explosives and DHAs are presented as inexpensive and impermanent fluorogenic indicators for the selective, standoff/remote identification of RDX and PETN.
2008
A new fluorescent turn-on probe (I) for the selective sensing and bioimaging of thiols is reported. In aq. buffer solns. at physiol. pH, thiols cleave the 2,4-dinitrobenzenesulfonyl group to release the red-emissive donor-acceptor fluorophore. The probe displays excellent immunity to interference from nitrogen and oxygen nucleophiles and the imaging of thiols in living cells is demonstrated. [on SciFinder(R)]
The tensile and compressive properties of triptycene-polycarbonates were tested over 6 orders of magnitude in strain rate. Initially we studied a low mol. wt., low triptycene content PC blended with Iupilon PC and then a series of higher mol. wt., higher triptycene content polymers. The PC blend with only 1.9 wt.% triptycene displayed up to 20% increase in modulus and up to 17% increase in yield strength with elongations over 80% as compared to the Iupilon PC. The higher mol. wt. T-PCs (up to 26 wt.% triptycene) exhibited improvements in modulus by over 20% and improvements in compressive strengths by nearly 50% at both low and high strain rates without any apparent sacrifice to ductility, as compared to Iupilon PC. All samples contg. triptycene units retained transparency and exhibited no signs of crystallinity or phase sepn. Moreover, both the blends and triptycene-PC copolymers displayed significantly altered dynamic mech. spectra, specifically, the emergence of a pronounced, new $\beta$' relaxation approx. 75° above the traditionally obsd. $\beta$ relaxation in PC (approx. -100°). The enhancement of the mech. properties obsd. provides valuable insights into the unique packing and interactions during plastic flow induced by the presence of triptycene units. [on SciFinder(R)]
A review on state-of-the-art vapor-phase solid-state chemosensing org. devices. The individual chem. sensing approaches and their transduction mechanisms are described. [on SciFinder(R)]
A one-step, one-pot assembly of arylethynylated cyclohexa-meta-phenylenes from meta-dibromide and meta-diboronates was accomplished using a 6-fold Suzuki macrocyclization, without the need for high diln. or slow-addn. techniques. [on SciFinder(R)]
To date, the cross effect (CE) and thermal mixing (TM) mechanisms have consistently provided the largest enhancements in dynamic nuclear polarization (DNP) experiments performed at high magnetic fields. Both involve a three-spin electron-electron-nucleus process whose efficiency depends primarily on two electron-electron interactions–the interelectron distance R and the correct electron paramagnetic resonance (EPR) frequency separation that matches the nuclear Larmor frequency, /omega(e2)-omega(e1)/ = omega(n). Biradicals, for example, two 2,2,6,6-tetramethyl-piperidine-1-oxyls (TEMPOs) tethered with a molecular linker, can in principle constrain both the distance and relative g-tensor orientation between two unpaired electrons, allowing these two spectral parameters to be optimized for the CE and TM. To verify this hypothesis, we synthesized a series of biradicals–bis-TEMPO tethered by n ethylene glycol units (a.k.a. BTnE)–that show an increasing DNP enhancement with a decreasing tether length. Specifically at 90 K and 5 T, the enhancement grew from approximately 40 observed with 10 mM monomeric TEMPO, where the average R approximately 56 A corresponding to electron-electron dipolar coupling constant omega(d)2 pi = 0.3 MHz, to approximately 175 with 5 mM BT2E (10 mM electrons) which has R approximately 13 A with omega(d)2 pi = 24 MHz. In addition, we compared these DNP enhancements with those from three biradicals having shorter and more rigid tethers-bis-TEMPO tethered by oxalyl amide, bis-TEMPO tethered by the urea structure, and 1-(TEMPO-4-oxyl)-3-(TEMPO-4-amino)-propan-2-ol (TOTAPOL) TOTAPOL is of particular interest since it is soluble in aqueous media and compatible with DNP experiments on biological systems such as membrane and amyloid proteins. The interelectron distances and relative g-tensor orientations of all of these biradicals were characterized with an analysis of their 9 and 140 GHz continuous-wave EPR lineshapes. The results show that the largest DNP enhancements are observed with BT2E and TOTAPOL that have shorter tethers and the two TEMPO moieties are oriented so as to satisfy the matching condition for the CE.
This Account details the use of building blocks known as iptycene units, which are particularly useful in the design of advanced materials because of their three-dimensional, noncompliant structures. Iptycenes are built upon [2,2,2]-ring systems in which the bridges are aromatic rings, and the simplest member of this class of compounds is triptycene. Iptycenes can provide steric blocking, which can prevent strong interactions between polymeric chromophores that have a strong tendency to form nonemissive exciplex complexes. Iptycene-containing conjugated polymers are exceptionally stable and display solution-like emissive spectra and quantum yields in the solid state. This application of iptycenes has enabled new vapor detection methods for ultratrace detection of high explosives that are now used by the U.S. military. The three-dimensional shape of iptycenes creates interstitial space (free volume) around the molecules. This space can confer size selectivity in sensory responses and also promotes alignment in oriented polymers and liquid crystals. Specifically, the iptycene-containing polymers and molecules align in the anisotropic host material in a way that minimizes the free volume. This effect can be used to align molecules contrary to what would be predicted by conventional models on the basis of aspect ratios. In one demonstration, we show that an iptycene polymer aligns orthogonally to the host polymer when stretched, and these structures approximate molecular versions of woven cloth. In liquid crystal solutions, the conjugated iptycene-containing polymers exhibit greater electronic delocalization, and the transport of excited states along the polymer backbone is observed. Structures that preserve high degrees of internal free volume can also be designed to create low dielectric constant insulators. These materials have high temperature stability (>500 degrees C) and hardness that make them potential interlayer dielectric materials for integrated circuits. In cases where the iptycene structures are less densely spaced along the polymer backbones, interlocking structures can be created. These structures allow for small interpolymer motions, but at large deformations, the steric clashes between iptycenes result in the transfer of load from one polymer to another. This mechanism has the ability to impart greater modulus, strength, and ductility. It is difficult to increase modulus without adversely affecting ductility, and classical high-modulus materials have low ductility. As a result, the use of interlocking iptycene structures is a promising approach to new generations of structural materials.
A review. [on SciFinder(R)]
Single-walled carbon nanotube films are fabricated from a homogeneous nanotube dispersion and a slow-evapn. technique. The transmittance and sheet resistance of the films are evaluated at room temp. Using nanotube films as conductive substrates for electrochem. deposition illustrates that these nanotube films can act as alternatives to indium tin oxide for optoelectronic applications. [on SciFinder(R)]
A chemiresistive material based on carbon nanotubes wrapped with a calixarene-substituted polythiophene displays a selective and sensitive response to xylene isomers, as demonstrated by conductance changes, quartz crystal microbalance, and fluorescence studies. This demonstrates the promise of low-cost, real-time sensors using host-guest chem. [on SciFinder(R)]
Poly(phenylene ethynylene)s bearing a high density of branched amphiphilic side-chains self-assemble at the air-water interface and in water-potassium dodecanoate-decanol lyotropic liquid crystals.
Near-infrared fluorescent probes for amyloid-beta (Abeta) are an exciting option for molecular imaging in Alzheimer's disease research and may translate to clinical diagnostics. However, Abeta-targeted optical probes often suffer from poor specificity and slow clearance from the brain. We are designing smart optical probes that emit characteristic fluorescence signal only when bound to Abeta.
A series of liq. crystals have been synthesized and studied based upon mononuclear ortho-platinated rod-like heteroarom. and 1,3-diketonate ligands. The liq. cryst. properties of these mols. were investigated using polarized light optical microscopy, differential scanning calorimetry, single crystal x-ray diffraction, and powder x-ray diffraction. Increasing the no. of alkyl chains attached to the 1,3-diketonate units resulted in a transition from lamellar (SmA) to hexagonal columnar phases (Colh). The 2-thienylpyridine units were previously unexplored in metallomesogenic complexes and these studies extend the utility of ortho-platinated 2-phenylpyridines and 2-thienylpyridines to produce columnar liq. cryst. phases. The platinum complexes all display photoluminescence and are of interest for electrooptical applications. [on SciFinder(R)]
The use of conjugated org. compds. in fluorescence sensing applications was demonstrated to provide an impressive method to detect energetic nitro-arom. compds. The amplified fluorescence quenching in conjugated polymers was used for trace detection of nitro-aroms. This method, under one-photon excitation, possesses certain limitations such as the use of harmful ultra-violet radiation and relatively high background noise from light scattering. A novel approach that utilizes the addnl. benefits of nonlinear optical methods involves multiphoton excited fluorescence. This technique employs IR excitation which is essential for eye-safety applications and allows for deeper penetration through the atm., with relatively low background noise. Two conjugated polymers are reported which show good multiphoton absorption properties. This is combined with the excellent sensitivity of the multiphoton excited fluorescence to the presence of tri-nitro toluene (TNT). The multiphoton absorption cross-sections are provided and the Stern-Volmer plots are discussed. This technique, in combination with the great analyte sensitivity of various org. materials, promises to be an important sensing technol. in the IR spectral regions. [on SciFinder(R)]
We report a chemiresistor that has been fabricated via simple spin-casting technique from stable CNT dispersion in hexafluoroisopropanol functionalized polythiophene. The sensor has shown high sensitivity and selectivity for a nerve reagent stimulant DMMP. A series of sensing studies, including field effect investigation, electrode passivation, and fluorescent measurement, indicate a combinative mechanism of charge transfer, introduction of scattering sites, and a configurational change of the polymer.
We report the syntheses of two monomers, 2,6-dibromotriptycene and 2,6-diiodotriptycene, and their applications in the homopolymn. via a nickel(0)-mediated Yamamoto-type polycondensation polymn., leading to a novel arom. polymer-poly(2,6-triptycene). This new polymer was characterized by 1H and 13C NMR spectroscopy, which exhibited an excellent match with the NMR spectra of the model compd., 2,2'-bitriptycene. In addn., the polymer was found to be highly sol. in common org. solvents, although it does not contain any flexible side chains. This good soly. was attributed to its high content of triptycene units, whose rigid three-dimensional structure was proposed to prevent dense packing of polymer chains. A highly transparent film could be obtained by spin-casting a chloroform soln. This polymer also demonstrated good thermal stability. [on SciFinder(R)]
The effect of added antioxidants and triplet quenchers on the photostabilities of thin films of a pentiptycene-contg. poly(p-phenylene ethynylene), and poly(9,9-dioctylfluorene) was studied. The rational design and synthesis of antioxidants and triplet quenchers that are compatible with conjugated polymer films are also presented. [on SciFinder(R)]
Heterocyclic monomers based on 2,1,3-benzooxadiazole and 2,1,3-benzothiadiazole bearing solubilizing side chains have been synthesized in high yields over three steps from readily available starting materials. The monomers are efficiently cross-coupled with diynes and bis(boronates) to afford high mol. wt. luminescent poly(arylene ethynylene)s and polyfluorenes that exhibit red-shifted absorption and emission maxima, greater soly., and reduced aggregation. [on SciFinder(R)]
Iptycene-type quinoxaline and thienopyrazine monomers were successfully synthesized via a condensation between 10-dihydro-9,10-ethanoanthracene-11,12-dione and the corresponding diamines. Copolymers based on fluorene and three iptycene monomers were prepd. via Suzuki coupling reaction, and they exhibited good soly. in appropriate org. solvents. These copolymers are fluorescent in both soln. and the solid state, emitting blue, greenish-blue, and red color due to the different electronic properties of the iptycene comonomers. The difference in their absorption and emission spectra was attributed to the donor-acceptor charge transfer interactions and/or polymer backbone conformational changes induced by steric effects. Moreover, the spectroscopic data clearly demonstrated the insulating effect of iptycene units, which prevented the aggregation of the polymer chains and the formation of excimers in the solid state. [on SciFinder(R)]
Hexafluoroisopropanol functionalized polythiophene is able to build up an isotropic self-supporting network structure. The gel network can be melted and then transformed via mech. shearing to form an anisotropic gel with the chains highly aligned along the shearing direction and the conjugated backbones $π$-stacked with respect to each other neighbors. The mechanism by which a dipole functionalized polythiophene can form a reversible network able to be deformed into structurally oriented films may be of interest in the development of novel processing routes for conjugated polymers. [on SciFinder(R)]
The synthesis and electrochem. properties of novel block copolymers are reported. Three different norbornene derivs. having phenylene-thiophene, phenylene-bithiophene, and phenylene-furan structures were copolymd. with either norbornene or 7-oxanorbornene derivs. via ring-opening metathesis polymn. (ROMP). Block copolymers' stabilities and solubilities could be improved by hydrogenation of double bonds in the polymer backbone. The block copolymers were subsequently cross-linked by anodic electropolymn. of phenylene-heterocycle moieties, affording conducting polymers. All of these three block copolymers were readily deposited on the electrode substrates, and their cyclic voltammograms (CVs) revealed an excellent reversibility in the redox cycles. [on SciFinder(R)]
The utility of Sato's titanium-mediated reduction of alkynes towards the synthesis of all cis-poly(phenylenevinylene)s (PPVs) is demonstrated by the syn-selective reduction of a variety of model diynes as well as a tetrayne. This technique was then applied to the reduction of a poly(phenyleneethynylene) (PPE) to provide the corresponding all-cis PPV polymer.
The syntheses and spectroscopic properties of eight new push-pull-type near-infrared fluorophores that contain either isobenzofuran or isothianaphthene subunits are presented. The isobenzofuran dyes demonstrate significantly red-shifted absorption compared with their isothianaphthene counterparts, which is attributed to isobenzofuran's more potent pro-quinoidal character.
An oligothiophene tweezer molecule, which has two quaterthiophene moieties connected to create an electrochemically activated hinge, has been synthesized. Two-electron oxidation of the tweezer molecule produces an intramolecular pi-dimer between the two oligothiophene moieties at room temperature as confirmed by UV-vis absorption, electrochemistry, and EPR experiments.
Stable pi-dimers are formed upon oxidation of the model units of proposed calix[4]arene-based molecular actuators in a solvent of low dielectric constant (CH 2Cl 2) at room temperature. Evidence from UV-vis, EPR, and DPV are all in agreement with the pi-dimer formation. In addition, pi-dimer formation is dependent upon the conformational flexibility of the calix[4]arene hinge.
2009
Carbon nanotube (CNT) compns. were prepd. by covalently grafting a Co(II) porphyrin to functionalized multiwalled carbon nanotubes (MWCNTs) via zwitterionic functionalization of the CNT sidewalls followed by a SN2 substitution reaction. The MWCNT-Co-porphyrin compns., mixed with Nafion, displayed excellent catalytic performance for oxygen redn. in acidic media (pH = 0.0-5.0) at room temp. With low catalyst loading, the oxygen redn. rates achieved are more than one order of magnitude higher than previously reported values for similar Co-porphyrin catalysts. These results demonstrate the advantages of systems of MWCNTs covalently linked to electrocatalytic mols. The electrodes are easily fabricated by a drop-casting vacuum drying procedure. Rotating disk electrode and rotating ring disk electrode measurements revealed the mechanism to be a direct four-proton and four-electron redn. of oxygen to water. These results demonstrate that new MWCNT electrocatalytic systems are potential substitutes for platinum or other metal-based cathode materials in proton conducting membrane fuel cells. [on SciFinder(R)]
A new polymerization technique that allows for the first-ever synthesis of poly(phenylenedicyanovinylene)s (PPCN2Vs) is described. PPCN2Vs, with their high electron affinities and structural versatility, seem ideally suited to address the need for new n-type polymers. Remarkably the polymers presented herein become more photoluminescent, in the thin film, under continuous irradiation.
A series of highly efficient, modular zwitterion-mediated transformations have been developed which enable diverse functionalization of carbon nanotubes (CNTs, both single-walled and multi-walled) and fullerenes. Three functionalization strategies are demonstrated. (1) Trapping the charged zwitterion intermediate with added nucleophiles allows a variety of functional groups to be installed on the fullerenes and carbon nanotubes in a one-pot reaction. (2) Varying the electrophile from dimethyl acetylenedicarboxylate to other disubstituted esters provides CNTs functionalized with chloroethyl, allyl, and propargyl groups, which can further undergo S(N)2 substitution, thiol addition, or 1,3-dipolar cycloaddition reactions. (3) Postfunctionalization transformations on the cyclopentenones (e.g., demethylation and saponification) of the CNTs lead to demethylated or hydrolyzed products, with high solubility in water (1.2 mg/mL for MWCNTs). CNT aqueous dispersions of the latter derivatives are stable for months and have been successfully utilized in preparation of CNT-poly(ethylene oxide) nanocomposite via electrospinning. Large-scale MWCNT (10 g) functionalization has also been demonstrated to show the scalability of the zwitterion reaction. In total we present a detailed account of diverse CNT functionalization under mild conditions (60 degrees C, no strong acids/bases, or high pressure) and with high efficiency (1 functional group per 10 carbon atoms for SWCNTs), which expand the utility of these materials.
Poly(pyridinium phenylene) conjugated polymers are synthesized by a cross-coupling and cyclization sequence. These polyelectrolytes are freely soluble in water and display high degrees of electroactivity. When reduced (n-doped) these materials display in situ conductivities as high as 160 S/cm. The high conductivity is attributed to the planar structure that is enforced by the cyclic structures of the polymer. The electron affinities are compared to PCBM, a C(60) based n-type material. We find that these polymers undergo excited state electron transfer reactions with other donor conjugated polymers and hence may find utility in photovoltaic devices.
Several fluorescent macrocycles based on 1,3-butadiyne-bridged dibenz[a,j]anthracene subunits have been synthesized via a multistep route. The synthetic strategy involved the initial construction of a functionalized dibenz[a,j]anthracene building block, subsequent installation of free alkyne groups on one side of the polycyclic aromatic framework, and a final cyclization based on a modified Glaser coupling under high-dilution conditions. Photophysical studies on three conjugated macrocycles revealed the formation of J-aggregates in thin films, as well as in concentrated solid solutions (polyisobutylene matrix), with peak absorption and emission wavelength in the range of lambda = 460-480 nm. The characteristic red-shifting of the J-aggregate features as compared to the monomer spectra, enhancement in absorption intensities, narrowed linewidths, and minimal Stokes shift values, were all observed. We demonstrate that improvements in spectral features can be brought about by annealing the films under a solvent-saturated atmosphere, where for the best films the luminescence quantum efficiency as high as 92% was measured. This class of macrocycles represents a new category of J-aggregates that due to their high peak oscillator strength and high luminescence efficiency have the potential to be utilized in a variety of optoelectronic devices.
Electroactive polymer (EAP) brushes with multiwalled carbon nanotube (MWCNT) backbones were prepd. by grafting nonconjugated polymers contg. pendant electroactive groups on the side walls of nanotubes via surface-initiated ring-opening metathesis polymn. Norbornene-functionalized MWCNTs were prepd. from 5-norbornene-2-methanol with pendant N,N,N',N'-tetramethyl-p-phenylenediamine. The ion-conducting monomer with pendant triethyleneoxymethyl ether groups was also synthesized by a similar reaction between norbornene-2,3-endo-dimethanol and triethylene glycol monomethyl ether tosylate. Block copolymer brushes with a shell of ion-conducting polymers surrounding the EAP were also prepd. These resultant electroactive materials exhibited high dispersibility in org. solvents and improved electroactivity and conductance, in comparison with the homo- and copolymers that were not attached to MWCNTs. [on SciFinder(R)]
We report an electrochem. transformation of binaphthols to give peri-xanthenoxanthene (PXX) groups in small mols. and within polymer backbones. The monomer 7,7'-bis(2,2'-bithiophen-5-yl)-1,1'-bi-2,2'-naphthol (2) was subjected to electropolymn., resulting in the segmented conducting polymer that is stable at low potentials. However, high-potential electrochem. oxidn. promoted cyclization of binaphthol units gives PXX, which transforms the moderately conducting segmented polymer into a highly conducting fully conjugated polymer. This oxidative cyclization is a highly effective means by which to incorporate a planar polycyclic heteroarom. structure (i.e., PXX) into thiophene-based conducting polymers. A model compd. study conclusively proved the proposed oxidative cyclization scheme. [on SciFinder(R)]
The synthesis and characterization of a biradical containing a 1,3-bisdiphenylene-2-phenylallyl (BDPA) free radical covalently attached to a 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) free radical are described. The synthesis of the biradical is a step toward improved polarizing agents for dynamic nuclear polarization (DNP).
2007
A series of nonaggregating carboxylate-functionalized poly(phenylene ethynylene)s (PPEs) have been synthesized for immobilization via electrostatic adsorption onto Eu3+-polystyrene microspheres with a mean diameter of 0.2 microm. This system is shown to constitute a ratiometric system that measures fluorescence quenching with high fidelity. The fluorescence quenching properties of the polymer-coated particles in response to methyl viologen and a naphthyl-functionalized viologen have been investigated in aqueous solutions to study the influence of electrostatic and hydrophobic interactions with pentiptycene-incorporated as well as macrocycle-containing polymers.
Degradation experiments and model studies suggested that the longer lived green fluorescence from an aggregated poly(p-phenylene ethynylene) (PPE) was due to the presence of highly emissive, low-energy, anthryl defect sites rather than the emissive conjugated polymer excimers proposed in a previous report. After elucidating the origin of the green fluorescence, additional anthryl units were purposely incorporated into the polymer to enhance the blue-to-green fluorescence color change that accompanied polymer aggregation. The improved color contrast from this anthryl-doped conjugated polymer led to the development of crude solution-state and solid-state sensors, which, upon exposure to water, exhibited a visually noticeable blue-to-green fluorescence color change.
Tert-butyl- and adamantyl-substituted alkene-bridged calix[4]arene monomers were synthesized by ring-closing metathesis (RCM). All the three possible conformers (cone, partial cone, and 1,3-alternate) were used as comonomers with cyclooctene and norbornene in ring-opening metathesis polymn. (ROMP). The resultant polymers were high-mol.-wt., transparent and stretchable materials with high calixarene incorporation (up to 25 mol% or 70 wt%) and low glass transition temps. [on SciFinder(R)]
Poly(phenylene ethynylene)s were designed and synthesized that are completely sol. in nematic liq. cryst. solvents at mol. wts. (GPC, relative to PS) of more than 100,000. These materials experience a chain extended rod conformation under these conditions with the polymer chains highly aligned with the liq. crystal director and an enhanced conjugation length detd. by optical spectroscopy. Structure-property relationships are investigated that reveal the role of steric congestion about the polymer mainchain in detg. the order parameter of the polymers. It was also found that the order polymer parameters increased with increasing mol. wts. [on SciFinder(R)]
An efficient, modular synthesis of triptycene derivatives is presented, in which the triptycene ring system is constructed from readily available anthraquinone and alkyne starting materials. A rhodium-catalyzed alkyne cyclotrimerization reaction serves as the key step in this new method for the preparation of these useful unnatural products.
Syntheses and spectroscopic properties of alkoxy-substituted para-acenequinones are reported. These compounds showed excellent alignment in nematic liquid crystals as evidenced by polarized UV-vis absorption and fluorescence measurements. [structure: see text]
Conjugated polymers undergo facile exciton diffusion. Different molecular structures were examined to study the role of the excited state lifetimes and molecular conformations on energy transfer. There is a clear indication that extended fluorescence lifetimes give enhanced exciton diffusion as determined by fluorescence depolarization measurements. These results are consistent with a strong electronic coupling or Dexter-type energy transfer as the dominating mechanism. The control of polymer conformations in liquid crystal solvents was also examined and it was determined that more planar conformations gave enhanced energy transfer to emissive low band-gap endgroups.
The electrochem. properties of conducting polymers are highly dependent on the microstructure. The authors report a method to produce specific microstructures of polypyrrole through electropolymn. in the presence of the amphiphile N-{11-(2-hydroxyethyldimethylammonium)undecanoyl}-N,N'-dioctyl-L-glutamate, bromide, which forms supramol. hydrogels with pyrrole in aq. soln. These hydrogels were used as templates during polypyrrole electropolymn. to give microstructures composed of the bundles of bilayer membranes. The highly porous nature of these films resulted in electrochem. properties superior to polypyrrole deposited under the same condition without use of an amphiphilic template. Anal. of the scan rate dependence on cyclic voltammogram reveals that the porous templated films facilitate fast diffusion of dopant ions. The actuation properties were also studied in aq. solns. contg. Na p-toluenesulfonate electrolyte. The strains displayed by the template polypyrrole films were twice those synthesized without the use of a template. [on SciFinder(R)]
The synthesis of new heterocyclic oligo(phenylene) analogues based on soluble, nonaggregating 1,2-diazines is reported. Improved palladium-catalyzed reductive coupling methods were developed to allow for the preparation of large quantities of iptycene-derived bipyridazines and biphthalazines, and the controlled synthesis of well-defined oligomers up to sexipyridazine. Crystallographic, spectroscopic, and computational evidence indicate that in these analogues, hindrance at the ortho position is relaxed relative to poly(phenylenes). The resulting building blocks are promising for incorporation in conjugated electronics materials, and as new iptycene-derived ligands for transition metals.
The incorporation of pendant iptycene units into polyesters creates a novel polymer-chain contour resembling \"mol. barbed wire.\". These types of units contain a unique structural property called the internal mol.-free vol. (IMFV) and have been shown to induce steric interactions between polymer chains through the minimization of the IMFV. This process creates a sterically interconnected polymer-chain network with high ductility because of two new mechanisms: mol. threading and mol. interlocking. The ability for these mechanisms to enhance the mech. properties of polyesters is robust across concn. and processing conditions. The size, shape, and concn. of these pendant units affect the mech. behavior, and results indicate that the larger units do not necessarily produce superior tensile properties. However, the mol.-barbed-wire architecture consistently produces enhanced mech. properties compared to the ref. polyester. The particular stress-strain response can be tailored by minute changes to the periphery of the iptycene unit. [on SciFinder(R)]
Skipping the monomer: A highly efficient reductive aromatization reaction transforms an unconjugated precursor polymer into a high-mol.-wt., butadiyne-linked anthracene homopolymer. Photophys. and electrochem. analyses reveal that some of these new materials display remarkable stability in both neutral and doped states and have unusually low intrinsic band gaps. [on SciFinder(R)]
A review. Conducting org. polymers, esp., polythiophene and substituted polythiophenes, for use as sensor materials are discussed. The charge transport mechanisms and response toward analytes based on functional groups and charge interactions are outlined. Sensor systems based on triaryl-Me carbocations are described. The design of specific mol. recognition centers to impart selectivity and the nature of the conducting polymer are also discussed. [on SciFinder(R)]
A review. In this review the authors restrict the discussions to purely fluorescence-based methods using conjugated polymers. Amplification, small ion sensing using amplifying fluorescent conjugated polymers (AFPs), AFPs for detection of explosives, conjugated polyelectrolytes as biosensors, AFPs for detection of small biomols., proteins, and DNA are discussed. [on SciFinder(R)]
The fluorescence-based detection of nonquenching, multicationic small molecules has been demonstrated using a blue-emitting, polyanionic poly(p-phenylene ethynylene) (PPE) doped with green-emitting exciton traps (anthryl units). Multicationic amines (spermine, spermidine, and neomycin) were found to effectively induce the formation of tightly associated aggregates between the polymer chains in solution. This analyte-induced aggregation, which was accompanied by enhanced exciton migration in the PPE, ultimately led to a visually noticeable blue-to-green fluorescence color change in the solution. The aggregation-based sensor exhibited poor sensitivity toward dicationic and monocationic amines, demonstrating that a conjugated polyelectrolyte sensor relying on nonspecific, electrostatic interactions may still attain a certain level of selectivity.
Ionic liquid crystals combine the unique solvent properties of ionic liquids with self-organization found for liquid crystals. We report a detailed analysis of the structure-property relationship of a series of new imidazolium-based liquid crystals with an extended aromatic core. Investigated parameters include length and nature of the tails, the length of the rigid core, the lateral substitution pattern, and the nature of the counterion. Depending on the molecular structure, two mesophases were observed: a bilayered SmA2 phase and the more common monolayered SmA phase, both strongly interdigitated. Most materials show mesophases stable to high temperatures. For some cases, crystallization could be suppressed, and room-temperature liquid crystalline phases were obtained. The mesomorphic properties of several mixtures of ionic liquid crystals were investigated. Many mixtures showed full miscibility and ideal mixing behavior; however, in some instances we observed, surprisingly, complete demixing of the component SmA phases. The ionic liquid crystals and mixtures presented have potential applications, due to their low melting temperatures, wide temperature ranges, and stability with extra ion-doping.
Single-walled carbon nanotubes (SWCNTs) and C60 are functionalized by reactions with di-Me acetylenedicarboxylate (DMAD) and alcs. mediated by 4-(dimethylamino)pyridine (DMAP) to give adducts such as the oxocyclopentenofullerene I. The reactions are proposed to occur by addn. of zwitterions generated from DMAP and DMAD to C60 or SWCNTs with concomitant ring closure followed by substitution of the (dimethylamino)pyridinium moiety of the intermediate adducts with alcs. Reaction of SWCNTs with DMAP, DMAD, and 1-dodecanol yields functionalized carbon nanotubes with significantly greater solubilities in org. solvents than unfunctionalized SWCNTs. The structure of I•CS2 is detd. by X-ray crystallog. [on SciFinder(R)]
A fluorescent chemosensor to detect satd. nitramine and nitrate ester explosives was devised based on a photochem. redn. reaction. 10-Methyl-9,10-dihydroacridine (AcrH2) was found to transfer a hydride ion equiv. to the high explosives RDX and PETN upon irradn. at 313 nm in degassed acetonitrile solns. Mechanistic photophys. studies indicate that the photoredn. of RDX proceeds via a two-step electron-hydrogen atom transfer reaction, whereas PETN photoredn. proceeds via a three-step electron-proton-electron transfer sequence. A zinc analog was synthesized and found to display an 80- or 25-fold increase in 480 nm emission intensity upon reaction with RDX or PETN, resp. Moreover, the Zn analog was found to be unresponsive to TNT and other common contaminants, in addn. to being photostable under ambient conditions. On the basis of these characteristics, a powerful chemosensor that displays a direct fluorescent response to either RDX or PETN is reported. [on SciFinder(R)]
Novel fluoride sensory systems were successfully developed. Previously developed method of the fluoride-induced lactonization to fluorescent mols. was detailed, and newly developed fluoride-induced arom. cyclization scheme was introduced. Based on the strategies using the specific affinity of fluoride to silicon, the authors' systems are highly selective for fluoride ion. Incorporation of the developed sensor to a conjugated polymer has successfully enhanced its sensitivity to fluoride ion. [on SciFinder(R)]
This paper describes a method to direct the formation of microstructures of poly(isobenzofuran) (PIBF) by chemical vapor deposition (CVD) on chemically patterned, reactive, self-assembled monolayers (SAMs) prepared on gold substrates. We examined the growth dependence of PIBF by deposition onto several different SAMs each presenting different surface functional groups, including a carboxylic acid, a phenol, an alcohol, an amine, and a methyl group. Interferometry, Fourier transform infrared (FT-IR) spectroscopy, X-ray photoelectron spectroscopy (XPS), gel permeation chromatography (GPC), and optical microscopy were used to characterize the PIBF films grown on the various SAMs. Based on the kinetic and the spectroscopic analyses, we suggest that the growth of PIBF is surface-dependent and may follow a cationic polymerization mechanism. Using the cationic polymerization mechanism of PIBF growth, we also prepared patterned SAMs of 11-mercapto-1-undecanol (MUO) or 11-mercaptoundecanoic acid (MUA) by microcontact printing (microCP) on gold substrates as templates, to direct the growth of the PIBF. The directed growth and the formation of microstructures of PIBF with lateral dimensions of 6 microm were investigated using atomic force microscopy (AFM). The average thickness of the microstructures of PIBF films grown on the MUO and the MUA patterns were 400 +/- 40 nm and 490 +/- 40 nm, respectively. SAMs patterned with carboxylic acid salts (Cu2+, Fe2+, or Ag+) derived from MUA led to increases in the average thickness of the microstructures of PIBF by 10%, 12%, or 27%, respectively, relative to that of control templates. The growth dependence of PIBF on the various carboxylic acid salts was also investigated using experimental observations of the growth kinetics and XPS analyses of the relative amount of metal ions present on the template surfaces.
2006
Heavy metal complexes that are phosphorescent at room temperature are becoming increasingly important in materials chemistry, principally due to their use in phosphorescent organic light-emitting devices (OLEDs). Their use in optical sensory schemes, however, has not been heavily explored. Homoleptic bis-cyclometalated Pt(II) complexes are known to undergo oxidative addition with appropriate electrophiles (principally alkyl halides) by either thermal or photochemical activation. We have applied this general reaction scheme to the development of a phosphorescence-based sensing system for cyanogen halides. To carry out structure-property relationship studies, a series of previously unreported Pt(II) complexes was prepared. Most of the complexes (excluding those that incorporated substituents on the ligands that forced steric crowding in the square plane) were strongly orange-red phosphorescent (Phi = 0.2-0.3) in a room-temperature oxygen-free solution. These sterically demanding ligands also accelerated the addition of cyanogen bromide to these complexes but slowed the addition of methyl iodide, indicating that the oxidative addition mechanisms for these two electrophiles is different. The lack of solvent-polarity effect on the addition of BrCN suggests a radical mechanism. Oxidative addition of BrCN to the metal complexes in solution or dispersed in poly(methyl methacrylate) gave blue-shifted emissive Pt(IV) complexes. The blue-shifted products give a dark-field sensing scheme that is in sharp contrast to energy transfer-based sensing schemes, which have limited signal-to-noise because of the presence of lower-energy vibronic bands of the energy donor that can overlap with the emission of the acceptor.
Crucial for the development of enhanced electrooptic materials is the construction of highly anisotropic materials. Nematic liquid crystals are able to control the chain conformation and alignment of poly(phenylene ethynylene)s (PPEs), producing electronic polymers with chain-extended planar conformations for improved transport properties. Here, we show that the dichroic ratio, and hence polymer alignment, increases dramatically when interpolymer interactions are introduced by end capping the PPE with hydrogen bonding groups. This increased order can be readily turned off by the introduction of a competing monofunctionalized hydrogen bonding compound. The formation of hydrogen bonds between the polymers results in the formation of gels and elastomers which may be of interest for future applications.
A series of poly(p-arylene butadiynylene)s containing zero, one, and two co-facial pi-pi interactions per repeat unit were synthesized and characterized. A surprisingly selective and high-yielding Diels-Alder cycloaddition of anthracene and nonsymmetric, sterically hindered anhydrides proved essential to generating the cofacial arene-containing monomers. Single-crystal X-ray structures display nearly parallel cofacial arenes that are within the van der Waals contact distances. The precursor molecules with cofacial arenes undergo reversible one- and two-electron oxidations to the radical cation and dication in CH2Cl2. The anhydrides were converted to N-alkyl imides to increase the solubility. High-molecular weight poly(p-arylene butadiynylene)s were prepared via Pd/Cu(I)/benzoquinone oxidative coupling of the diacetylene monomers. The resulting polymers are highly emissive in solution and thin films. The ionization potentials were measured using ultraviolet photoelectron spectroscopy with thin films. Last, fluorescence measurements of polymer thin films during continuous irradiation indicate that the most hindered polymer is more resistant to photobleaching.
In a previous publication, we described the use of biradicals, in that case two TEMPO molecules tethered by an ethylene glycol chain of variable length, as polarizing agents for microwave driven dynamic nuclear polarization (DNP) experiments. The use of biradicals in place of monomeric paramagnetic centers such as TEMPO yields enhancements that are a factor of approximately 4 larger (epsilon approximately 175 at 5 T and 90 K) and concurrently the concentration of the polarizing agent is a factor of 4 smaller (10 mM electron spins), reducing the residual electron nuclear dipole broadening. In this paper we describe the synthesis and characterization by EPR and DNP/NMR of an improved polarizing agent 1-(TEMPO-4-oxy)-3-(TEMPO-4-amino)propan-2-ol (TOTAPOL). Under the same experimental conditions and using 2.5 mm magic angle rotors, this new biradical yields larger enhancements (epsilon approximately 290) at lower concentrations (6 mM electron spins) and has the additional important property that it is compatible with experiments in aqueous media, including salt solutions commonly used in the study of proteins and nucleic acids.
The dynamic transfer of a conjugated polymer's organization-dependent properties from the solution state to the solid film state was probed by circularly polarized luminescence (CPL) and circular dichroism (CD) spectroscopy. Different supramolecular organizations within films and aggregate solutions of a chiral poly(p-phenylenevinylene) derivative led to opposite CPL and CD spectra. These dramatic property differences were controlled by regulating the polymer's self-assembly through solvent selection and film annealing. Therefore, different processing conditions can greatly affect the functional properties of conjugated polymer films employed in various optoelectronic applications.
Derivatives of relatively electron rich 1,5-dialkoxynaphthalene (Dan) donors and relatively electron deficient 1,4,5,8-naphthalenetetracarboxylic diimide (Ndi) acceptors have been exploited in the folding and self-assembly of a variety of complex molecular systems in solution. Here, we report the use of Dan and Ndi derivatives to direct assembly of extended columns with alternating face-centered stacked structure in the solid state. A variety of 1:1 Dan:Ndi mixtures produced mesophases that were found to be stable over temperature ranges extending up to 110 degrees C. Analysis of these mesophases indicates mixtures with soft/plastic crystal phases and a few mixtures with the thermodynamic properties of true liquid crystals, all composed of alternating donor-acceptor columns within. Importantly, a correspondence was found between the clearing and crystallization points of the mesophase mixtures and the melting/clearing points of the component Ndi and Dan units, respectively. This correspondence enables the predictable tuning of mesophase phase transition temperatures. The study of sterically hindered derivatives led to a set of mixtures in which a dramatic and sudden color change (deep red to yellow) was observed upon crystallization of the mesophase due to a phase separation of the component donor and acceptor units.
A series of 2,5-thiophene-substituted 1',2',3',4',5'-pentamethylazaferrocene complexes were synthesized and electropolymerized to produce polymers with fully pi-conjugated backbones. The length and hence oxidation potential of the conjugated linker (the thiophene fragments) between the metal centers were varied to understand the influence of the metal-metal interactions on the overall electroactivity of the resulting polymer. These complexes were electrochemically polymerized, and the resulting polymers were characterized by cyclic voltammetry, in situ conductivity, and spectroelectrochemistry measurements. The iron-centered oxidations significantly increased the conductivity of the polymer. The results reveal that shorter conjugated linkers cause the onset of conductivity to occur at lower potentials. This effect implies that a superexchange mechanism is likely operative in the charge migration of these polymers.
Sol. anthryl-based conjugated poly(phenylene ethynylene)s (PPEs) have been synthesized using palladium-catalyzed Sonogashira-Hagihara cross-coupling polymn. reactions. Mol. wts. up to 3.5 × 104 g•mol-1 were obtained, making them suitable for spectroscopic soln. characterizations and thin film processing. The selective reactivity of these polymers as multidienes has been successfully demonstrated with strong dienophiles. Diels-Alder reactions proceed cleanly to completion with unhindered dienophiles such as N-alkylated maleimide derivs. TGA anal. revealed thermal retro-Diels-Alder reactions at modest temps. around 210 °C. Compared with their parent polymers, the cycloadduct polymers exhibited dramatic hypsochromic shifts of their emission and absorption maxima up to 80 nm along with a considerable quantum yield enhancement. These original anthryl-based polymers appear attractive as reactive conjugated materials whose optical properties can easily be tuned with quant. Diels-Alder reactions. [on SciFinder(R)]
This paper reports water-sol. viologen quenchers that bear hydrophobic substituents and describes their effectiveness as quenchers of emission from thin films of hydrophobic, water-insol. poly(p-phenylene ethynylene)s. [on SciFinder(R)]
The effect of linker length on biol. recognition at the air-water interface using energy transfer between a fluorescent conjugated polymer and dye-labeled protein. A longer linker provided greater binding of the protein as evidence by the dramatically increased energy transfer. This may be due to an increased hydrophilicity of the side chain, which allowed the biotin to better access the streptavidin located in the aq. subphase. Subtle changes in the polymer structure can thus have important consequences for analyte recognition. [on SciFinder(R)]
Tropone-Contg. thiophene monomers were prepd. and electrochem. polymd. under ambient conditions to form polymers. The polymers with various concns. of TFA were studied by CV, UV-vis-NIR, and cond. In conclusion, the cond. of tropone-contg. polythiophene can be switched reversibly by a protonation/deprotonation process. The cond. switching mechanism of this polymer is alternative to that of conventional proton dopable polymers such as polyaniline. [on SciFinder(R)]
Convenient syntheses of 9,9-bis(trifluoromethyl)fluorene and 6,6,12,12-tetrakis(trifluoromethyl)indenofluorene are reported. The monomers were readily prepd. in two steps from simple halo-aroms. and hexafluoroacetone, which serves as the source of the geminal trifluoromethyl groups. Iodination yields polymerizable monomers that were used to prep. several conjugated polymers. The photophys. properties of these polymers are reported. The polymers demonstrate slightly blue-shifted UV-vis absorption and fluorescence emission spectra and high soln. and solid-state fluorescence quantum efficiency. Polymer photobleaching expts. reveal that poly(fluorene)s with geminal trifluoromethyl substituents demonstrate greater photooxidative stability than poly(9,9-dioctylfluorene). [on SciFinder(R)]
A report on a water-sol. photooxidizing amplifying fluorescent polymer (AFP) and its performance in photoinduced electron transfer (PET) based detection of amino acids, neurotransmitters, and proteins possessing electron-donating arom. moieties in aq. buffer is presented. This report has shown that a water-sol. photooxidizing AFP could serve as a PET-based sensor for amino acids, neurotransmitters and proteins with electron-donating arom. compds. in aq. media. The results show that the quenching efficiency is strongly dependent on electrostatic interaction of the anionic polymer and quencher as well as hydrophobic and electron-transfer interactions between the polymer chains and quencher. [on SciFinder(R)]
This paper describes the synthesis and properties of poly(isobenzofuran) (PIBF) films prepd. by a thermal chem. vapor deposition (CVD) process. The synthesized precursor monomer, 1,2,3,4-tetrahydro-1,4-epoxynaphthalene, is pyrolyzed by flowing it through a tube furnace at temps. of 600-750°. A reactive intermediate, isobenzofuran (IBF) monomer, is produced by this pyrolysis and deposited onto a silicon substrate where it polymerizes to form a thin coating of poly(isobenzofuran) (PIBF) on the surface. The chem. structure and compn. of the PIBF films are supported by NMR spectroscopy, Fourier transform IR spectroscopy (FT-IR), and XPS. The wt.-av. mol. wt. of the PIBF films range from 5500 to 9400 by varying the deposition (stage), pyrolysis (furnace), and vaporization (source) temps. With the variation of deposition temp. (5, 10, 15, 20, 25°) and pyrolysis temp. (600, 650, 700, 750°), significant changes are obsd. in deposition (growth) rate, mol. wt., and morphol. while the chem. structure of the PIBF films remain the same as probed by FT-IR and XPS anal. On the other hand, variation of the vaporization temp. (40, 45, 50, 55, 60°) leads to significant changes in the chem. structure as well as in the deposition rate, mol. wt., film uniformity, and morphol. By exploring several operating conditions, we have obtained optimal conditions for deposition temp. (10°), pyrolysis temp. (750°), and vaporization temp. (60°) that provide good film properties as well as fast film growth. To investigate the possible role of cationic initiation in IBF polymn., PIBF films were deposited on several surfaces tailored with self-assembled monolayers (SAMs) of thiols that have functional groups of different acidities, including a carboxylic acid (-COOH), a phenol (-PhOH), an alc. (-OH), an amine (-NH2), and a Me group (-CH3). We obsd. the fastest growth of PIBF (k = 2.5 \AA/s) on the carboxylic acid-terminated surfaces whereas the slowest growth was on the methyl-terminated surfaces (k = 0.02 \AA/s). On the basis of the exptl. observations, we proposed a growth mechanism for the PIBF films by the CVD process. [on SciFinder(R)]
A self-polymerizable AB-type monomer for Diels-Alder (D-A) polymn. was prepd., and its polymn. was carried out in the melt phase and at high pressure in soln. The former method generated only low-mol.-wt. polymer, but the latter one offered an efficient polymn. with increased mol. wt., due to the effect of high pressure on reactions with a neg. activation vol. A pyridinium p-toluenesulfonate-catalyzed dehydration reaction of the D-A polymer led to a novel arom. ladder polymer, poly(iptycene), which is sol. in common org. solvents and stable up to 350 °C. The NMR and UV-vis spectra of these polymers match the spectra of their corresponding model compds., the synthesis of which is also reported. [on SciFinder(R)]
The prepn. and mech./structural characteristics were studied of a polyester contg. 21% triptycene monomer, vs. a ref. polyester homolog wherein benzene replaces the triptycene residue. Solvent-cast films and tension heat-treated (THT) films were studied by tensile deformation and wide-angle x-ray scattering. The addn. of triptycene units increases the Tg and, contrary to what is typically obsd., also increases the ductility of film samples. In comparison to the solvent-cast non-triptycene polyester films, the triptycene polyester films displayed a nearly 3-fold increase in Young's modulus, an approx. 3-fold increase in strength, and a more than 20-fold increase in strain to failure. THT films of the triptycene polyester exhibited a modulus more than 7 times that of the non-triptycene as-cast polyester and strength greater than 14 times higher for roughly the same strain to failure. This unusually beneficial mech. behavior is primarily attributed to the ability of individual triptycene units to express what was termed as internal mol. free vol. (IMFV). The triptycene polymers adopt favorable conformations that minimize the IMFV, and the resultant assembly introduces two mechanisms for the enhancement of tensile mech. properties: mol. threading and mol. interlocking. [on SciFinder(R)]
Poly(phenyleneethynylene)s (PPEs) capable of reacting with thiol-contg. mols. have been designed and synthesized with no.-av. mol. wts. ranging from 8000 to 11,000. The PPEs contain pendent 3a,4,7,7a-tetrahydro-4,7-epoxy-1H-isoindole-1,3(2H)-dione groups available to participate in conjugate addn. and Diels-Alder chem. after being thermally activated. If the maleimide group is left unmasked during palladium-catalyzed cross-coupling polymn., it leads to side reactions and the prodn. only of short chain oligomers (Mn < 3000, DP ∼ 4). Reversible Diels-Alder reactions between the maleimides and furan were detd. to be a very effective method to produce a masked Michael acceptor that can be unveiled after polymn. under relatively mild thermal conditions. Cycloreversion to the maleimide has been monitored by thermogravimetric anal. (TGA), TGA-MS, IR, and NMR. A PPE contg. the masked maleimide unit has been modified with a thiolated carboxy-X-rhodamine (ROX) dye, and the resulting absorbance and fluorescence spectra as well as gel permeation chromatograms (GPC) are presented. [on SciFinder(R)]
We have synthesized high-mol.-wt. main-chain calixarene homopolymers via linkages on lower rims. We have found that the calixarene conformation has a profound influence on the polymn. Our synthetic methodol. can be applied to the polymns. of other sterically demanding and conformationally flexible monomers. Main-chain calixarene polymers linked through the lower rims present new opportunities to create efficient sepns., and materials for sensing and actuation, as a result of their dynamic receptor properties. [on SciFinder(R)]
[reaction: see text] The syntheses of a series of fluorine- and alkyl/alkoxy-functionalized tetracenes are reported. These functional groups are found to improve the solubility in common organic solvents and tune molecular arrangement in solids. The crystal packing, electrochemical behavior, and UV-vis absorbance spectroscopy of these materials are discussed.
To understand the charge transport ability of metal coordinated cyclobutadiene, a series of cyclobutadiene cobalt cyclopentadiene (CbCoCp) complexes contg. electrochem. polymerizable 3,4-ethylenedioxythiophene units were synthesized. The complexes were electrochem. polymd. and the resulting polythiophenes were characterized by cyclic voltammetry, in situ cond. and UV-vis spectroelectrochem. Several different derivs. of the CbCoCp complexes and a model study suggested that if the oxidn. of the org. fragment was above CoI/II redox couple of the CbCoCp complex, detrimental side reactions occurred. Side reactions did not occur if the oxidn. potential of the org. fragment was below the oxidn. potential of the metal. [on SciFinder(R)]
Exposure of hydrazine vapor to conjugated-polymer films reduces the small no. of oxidized trap sites that quench excitons. This scheme is effective for trace hydrazine detection with a detection limit of <1 ppm. The introduction of an oxidizing dopant quenches the polymer emission and permits detection of hydrazine with larger on/off ratios. [on SciFinder(R)]
We describe hot-filament chem. vapor deposition of polyisobenzofuran (PIBF) films and characterize their chem. structure and surface morphol. The precursor monomer, 1,2,3,4-tetrahydro-1,4-epoxy-naphthalene, is pyrolyzed by flowing it over an array of hot filaments held at three different temps. (680, 738, and 800 °C). The produced intermediate, iso benzofuran , is deposited onto a silicon substrate as thin films of PIBF. Fourier transform IR spectroscopy and XPS revealed that the films prepd. at 800 °C possess a chem. structure and a compn. different from those prepd. at lower filament temps. (680 and 738 °C). Increasing filament temp. also leads to the formation of defect domains in the polymer films. By at. force microscopy, we observe a decrease in surface roughness and in the av. size of polymer grains in PIBF domains as the filament temp. is increased. Defect domains exhibit a rougher surface as well as larger size polymer grains than those in PIBF domains. Spectroscopic and microscopic results suggest that growth of the polymer in the defect domains proceeds by a mechanism different from that in the PIBF domains. [on SciFinder(R)]
Nitric oxide (NO) was implicated in a wide range of biol. processes including cellular signaling, coronary artery dilation, immune system response, and neurotransmission. With this in mind, the development of direct, sensitive, and selective sensing schemes for NO has attracted great interest in recent years. The development of a selective, ppm level, gas-phase NO detection system based on chemoresistive changes in a cobalt-contg. metallopolymer film device is discussed. These films are easily prepd. and elec. interfaced, and showed detection limits of NOx <1 ppm. [on SciFinder(R)]
The in situ formation of well-dispersed silver nanoparticles in an insulating polymer matrix is reported. Poly(aryl ether)s (PAEs) incorporating an oxadiazole moiety were synthesized, and they demonstrated selective interaction with silver ions. Polymer-ion thin films were easily spin cast and exposed to hydrazine vapors to induce metal nucleation. Introducing a triptycene monomer into the polymer backbone resulted in a highly porous matrix and prevented nanoparticle aggregation. This provides a generalized approach to the facile prepn. of well-dispersed metal nanoparticles embedded thin films. [on SciFinder(R)]
2005
Poly(p-phenylenevinylene)s containing trifluoromethyl substituted aromatic rings (CF3-PPVs) exhibit high photooxidative stability to give robust materials suitable for demanding applications.
Recent reports of highly conductive metallopolymers are reviewed. This literature is classified into one of two categories (inner or outer sphere) depending on the mode of interaction between the transition metal centers with each other and the conducting polymer backbone. The critical nature of charge transport is discussed in the context of the relative energies of the organic polymer-based and metal-centered redox processes. Also included are recent advances in the development of functional materials based on metal-containing conducting polymers.
Structural and electronic effects on the efficiency of DMNB detection with fluorescent conjugated polymers are described.
Rotaxane structures which provide functional insulation to conducting polymers can provide over a 4000-fold redn. in cond. over non-rotaxane structures and with electronically active copper ions in the rotaxane units the cond. increases by more than 80 times relative to the metal-free analog. [on SciFinder(R)]
We investigate the effects of thermodynamical variables, intermol. interactions and block lengths on phase and orientational ordering of guest tri-block co-polymers in a host glassy matrix of short mol. rods. The A and B blocks can align to the short rod mols. Using a field theoretic formulation we demonstrate the occurrence of a nematic-nematic (N/N) first order transition from a guest stabilized to a guest-host stabilized region, a reentrant transition from a guest stabilized nematic region to a host only stabilized regime via an isotropic phase and the possibility to selectively stabilize the orientation of the A or/and B blocks. [on SciFinder(R)]
The design, synthesis, and electropolymn. of sterically hindered pyrrole derivs. are described. Endo and exo adducts of cyclopentadiene and N-phenylmaleimide were converted to give bicyclo[2.2.1]heptane-fused pyrrole monomers, in which a Ph group is rigidly placed proximate the pyrrole fragment. Oxidative polymn. of these monomers affords highly conductive polypyrroles. The rigid mol. scaffold of the pyrrole monomers limits cross-communication between adjacent conducting strands and produces a defined narrow potential window of high cond. dominated by intrachain polaronic charge carriers. The resultant loosely packed polymer chains allow for superior sensory properties. Notably, the elec. cond. of oxidatively doped poly(7) could be reversibly modulated in aq. electrolytes at pH 3-9. [on SciFinder(R)]
Current methods for myelin staining in tissue sections include both histological and immunohistochemical techniques. Fluorescence immunohistochemistry, which uses antibodies against myelin components such as myelin basic protein, is often used because of the convenience for multiple labeling. To facilitate studies on myelin, this paper describes a quick and easy method for direct myelin staining in rodent and human tissues using novel near-infrared myelin (NIM) dyes that are comparable to other well-characterized histochemical reagents. The near-infrared fluorescence spectra of these probes allow fluorescent staining of tissue sections in multiple channels using visible light fluorophores commonly used in immunocytochemistry. These dyes have been used successfully to detect normal myelin structure and myelin loss in a mouse model of demyelination disease.
The synthesis and optical properties of several phosphorescent conjugated poly(phenylene)s contg. cyclometalated square-planar platinum (II) complexes are reported. These electronic polymers were synthesized via Suzuki cross-coupling of a dibromophenylpyridine-ligated Pt(ii) complex with a fluorene diboronic ester. Their optical properties are characterized by relatively strong orange room-temp. phosphorescence with well-resolved vibronic structure in both frozen 2-methyltetrahydrofuran glass and room-temp. fluid soln. Time-resolved phosphorescence spectroscopy has shown that the polymers have excited state lifetimes of approx. 14 $μ$s. These optical properties of the oligomers and polymers are contrasted with those of small model complexes, the optical properties of which have a strong dependence on the identity of the $\beta$-diketonate ligand used. The potential utility of phosphorescent conjugated polymers is illustrated by examn. of the diffusive quenching due to oxygen as a function of mol. structure. [on SciFinder(R)]
We report the synthesis and photophysical investigation of a series of rotaxanes in which the physical confinement of the donor and acceptor (DA) pair leads, in some cases, to emissive exciplexes. As a comparison, we examined the photoinduced charge-transfer processes in the same DA mixtures under intermolecular conditions. The interlocked configuration of the rotaxane facilitates pi orbital overlap of the excited state DA pair by keeping their center-to-center distance extremely small. This increased interaction between the DA pair significantly lowers the activation energy for exciplex formation (E(a)) and helps stabilize the highly polar charge-transfer complex. We find that the stabilizing effect of the rotaxane architecture compensates for the modest thermodynamic driving force for some charge-transfer interactions. In addition, we examined the temperature dependence on the rotaxanes' optical properties. Metal coordination to the tetrahedral cavity disrupts the cofacial conformation of the DA pair and quenches the fluorescence. Binding of alkali metal ions to the 3,4-ethylenedioxythiophene (EDOT)-based rotaxane, however, gives rise to the emergence of a new weak emission band at even lower energies, indicative of a new emissive exciplex.
A set of carboxylate-functionalized poly(phenylene ethynylene)s (PPEs) has been synthesized in which the carboxylic acid groups are separated from the polymer backbone by oligo(ethylene glycol) spacer units. These polymers are soluble in water and organic solvents and have photophysical properties that are sensitive to solvent conditions, with high salt content and the absence of surfactant promoting the formation of aggregates of relatively low quantum yield and long fluorescence lifetime. Quenching of these materials by the dinitrophenyl (DNP) chromophore (K(SV) approximately 10(4)) is also highly solvent-dependent. The presence of carboxylate groups far from the polymer backbone appended to each repeating unit allows for the postpolymerization modification of these PPEs with peptides by methods analogous to those described for carboxylate-functionalized small-molecule dyes. Covalent attachment of the fluorescence-quenching 14-mer Lys(DNP)-GPLGMRGLGGGGK to the PPE results in a nonemissive substrate whose fluorescence is restored upon treatment with trypsin. The rate of fluorescence turn-on in this case is increased 3-fold by the presence of surfactant, though the actual rate of peptide hydrolysis remains the same. A small-molecule mimic of the polymer-peptide system shows a smaller fluorescence enhancement upon treatment with trypsin, illustrating the value of polymer-based amplification in this sensory scheme.
We describe herein a polymeric material that prefers to align perpendicular to a stretch-aligned polymer host in the solid state. Poly(iptycene) poly-1 was synthesized from monomer 1 under hyperbaric techniques via a Diels-Alder polymerization. Polarized excitation spectra of the anthracene end groups in this material in a stretch-aligned, solution-cast poly(vinyl chloride) (PVC) film showed that the poly(iptycene) prefers to align normal (counter aspect ratio) to the stretching direction of the PVC. This is explained by a \"threading\" mechanism, whereby the PVC intercalates through the internal free volume presented by poly-1, similar to effects observed in small molecule iptycenes under similar conditions.
Conjugated polymers often display a decrease of fluorescence efficiency upon aggregation due in large part to enhanced interpolymer interactions that produce weakly emissive species generally described as having excimer-like character. We have found that poly(phenylene ethynylene)s with fused pendant [2.2.2] ring structures having alkene bridges substituted with two ester groups function to give highly emissive, broad, and red-shifted emission spectra in the solid state. To best understand the origin of this new solid-state emissive species, we have performed photophysical studies of a series of different materials in solution, spin-coated thin films, solid solutions, and Langmuir films. We conclude that the new, red-shifted, emissive species originate from excimers produced by interchain interactions being mediated by the particular [2.2.2] ring system employed. The ability to design structures that can reliably produce highly emissive conjugated polymer excimers offers new opportunities in the emission tailoring of electroluminescence and sensory devices.
We report the synthesis of a series of poly(p-phenylene ethynylene)s (PPEs) with high ionization potentials and associated high excited-state electron affinities. Their photophysical properties were investigated using steady-state and time-resolved fluorescence techniques. The ionization potentials of the polymer thin films were determined using ultraviolet photoelectron spectroscopy (UPS), and those with the highest ionization potentials displayed high sensitivity for the detection of electron-donating aromatic compounds. The effects of sterics, chemical structure, and electronic properties on the polymers' sensory responses were investigated by fluorescence quenching experiments in both solution and solid thin films. In addition, we report that in some cases the excited-state charge-transfer complexes (exciplexes) of the PPEs with analytes were observed. These latter effects provide promising opportunities for the formation of sensitive and selective chemical sensors.
Efficient energy migration in conjugated polymers is critical to their performance in photovoltaic, display, and sensor devices. The ability to precisely control the polymer conformation is a key issue for the experimental investigations and deeper understanding of the nature of this process. We make use of specially designed iptycene-containing poly(p-phenylene ethynylene)s that display chain-extended conformations when dissolved in nematic liquid crystalline solvents. In these solutions, the polymers show a substantial enhancement in the intrachain exciton migration rate, which is attributed to their increased conjugation length and better alignment. The organizational enhancement of the energy transfer efficiency, as determined by site-selective emission from lower energy traps at the polymer termini, is accompanied by a significant increase of the fluorescence quantum yield. The liquid crystalline phase is a necessary requirement for these phenomena to occur, and when the temperature was increased above the nematic-isotropic transition, we observed a dramatic reduction of the energy transfer efficiency and fluorescence quantum yield. The ability to improve the exciton migration efficiency through precise control of the polymer structure with liquid crystalline solutions demonstrates the importance of a polymer's conformation for energy transfer, and provides a way to improve the energy transporting performance of conjugated polymers.
We have studied the lasing characteristics from a dye-doped nematic liq. crystal layer sandwiched by two polymeric cholesteric liq. crystal films as photonic band gap materials. The nematic layer possessing birefringence brings about the following remarkable optical characteristics: (1) reflectance in the photonic band-gap (PEG) region exceeds 50 %, due to the retardation effect, being unpredictable from a single CLC film; (2) efficient lasing occurs at the notch of PEG; (3) the lasing emissions contain both right- and left-circular polarizations. In this study, we demonstrate that a 100-$μ$m-thick nematic liq. crystal layer system shows several dips, resulting in multi-mode lasing actions just at the dips within PBG. [on SciFinder(R)]
A new series of poly(p-phenylenebutadiynylene)s has been synthesized with unique polymer structural features. In these systems each of the p-phenylene units in the conjugated backbone is the core of a rigid three-dimensional iptycene scaffold. The fluorescence quenching properties of these polymers in response to a series of electron-deficient arom. compds. have been investigated in both soln. and the solid state. It was found that in soln. these polymers displayed higher quenching sensitivity toward studied quenchers compared to a more open-structure iptycene-contg. poly(p-phenyleneethynylene). The quenching behaviors of the conjugated polymer were shown to be strongly influenced by the configuration of the incorporated iptycences. The thin films investigations revealed differences in both the fluorescence quenching and the recovery processes. Distinct behaviors indicated that the fluorescence quenching in the solid state is dictated by different factors than those in soln. Our results further suggest that poly(p-phenylenebutadiynylene)s contg. large iptycene scaffolds that introduce porosity have the ability to efficiently sequester the quencher mols. within thin films as these materials display slow fluorescence recoveries. [on SciFinder(R)]
Several poly(phenylene-ethynylene)s with pendant hexafluoro-2-propanol (HFIP) groups have been synthesized and characterized in terms of their soln. and thin-film optical properties. The incorporation of strongly hydrogen-bond-donating HFIP groups into conjugated polymers is shown to greatly enhance their fluorescence response upon exposure to the vapors of several hydrogen-bond-accepting analytes such as pyridine and 2,4-dichloropyrimidine. The enhanced sensitivity of these conjugated polymer-based chemosensors is the result of stronger analyte/polymer binding interactions and more facile photoinduced charge-transfer reactions with hydrogen-bonded analytes. [on SciFinder(R)]
Alternating polyelectrolyte \"layer-by-layer\" deposition was used to create emissive thin films of a weakly anionic, nonaggregating poly(phenylene ethynylene) on silica microspheres. The resulting particles are smooth and emit fluorescence solely from the outside polymer-coated surface. Suspensions of the coated microspheres show similar fluorescence properties to polymers in soln., but with enhanced sensitivity (up to 200-fold) to nitroarom. quenchers. These enhancements are attributed to a combination of surface and electronic effects. [on SciFinder(R)]
Two isomeric polymers, which contain meta- or para-phenylene linkages between conducting segments, were synthesized and compared by electrochem. methods. The nonconjugated poly(1,5-diacetoxy-m-phenylene tetrathienylene) (PMPT-OAc) showed similar electroactivity to the para-isomer, poly(2,5-diacetoxy-p-phenylene tetrathienylene) (PPPT-OAc), in the cyclic voltammetry and in-situ cond. measurements. Spectroelectrochem. showed a similar buildup of sub-band-gap electronic transitions. Deacetylation of the polymers was performed successfully by reaction with hydrazine, producing PMPT-OH and PPPT-OH. Both phenol-substituted polymers exhibited greater electroactivity than the acetoxy-substituted polymers. In addn., the phenol substituents were found to lower the \"turn-on\" potential in the cond.-potential profile for PMPT-OH, but not for PPPT-OH. An alkoxy-substituted PMPT-OMe shows electroactivity similar to that of PMPT-OAc in the cyclic voltammetry, in-situ cond., and spectroelectrochem. [on SciFinder(R)]
CD spectroscopy was used to demonstrate that a film spin-cast from a polymer having a polyphenylenevinylene backbone and enantiomerically pure chiral side chains can be fabricated from different solvents to have correspondingly different chiral architectures with opposite handedness. [on SciFinder(R)]
Conjugated polymers (CPs) contg. amino groups have been synthesized, and their optical properties in both soln. and thin film have been studied. New monomers required for the synthesis of these polymers have been readily prepd. via efficient synthetic routes. These monomers have been successfully polymd. with a variety of comonomers. The spectral positions of the absorption and emission spectra correlate with the degree of electron d. on the polymer chain. Polymers contg. N-alkylcarbazole units display similar optical properties in soln. to most CPs. Polymers with dialkylamino groups, however, display very different optical properties, including broadened absorbance and emission spectra, larger Stokes shifts, and longer excited state lifetimes. These results are consistent with a significant difference between the mol. geometries of the absorbing and emitting states. The solid state emission of most of the polymers is sufficient to warrant them for consideration as fluorescent sensing materials. [on SciFinder(R)]
Blue poly(phenylene-ethynylene) (PPE) electroluminescence is achieved in a single layer organic light emitting device. The polymeric system consists of an oxadiazole grafted PPE, which combines the necessary charge transport properties while maintaining the desirable efficient, narrow light-emitting properties of the PPE. Incorporation of a pentiptycene scaffold within the PPE structure prevents ground-state and excited-state interactions between the pendent oxadiazole units and the conjugated backbone.
Societal needs for greater security require dramatic improvements in the sensitivity of chemical and biological sensors. To meet this challenge, increasing emphasis in analytical science has been directed towards materials and devices having highly nonlinear characteristics; semiconducting organic polymers (SOPs), with their facile excited state (exciton) transport, are prime examples of amplifying materials. SOPs have also been recognized as promising lasing materials, although the susceptibility of these materials to optical damage has thus far limited applications. Here we report that attenuated lasing in optically pumped SOP thin films displays a sensitivity to vapours of explosives more than 30 times higher than is observed from spontaneous emission. Critical to this achievement was the development of a transducing polymer with high thin-film quantum yield, a high optical damage threshold in ambient atmosphere and a record low lasing threshold. Trace vapours of the explosives 2,4,6-trinitrotoluene (TNT) and 2,4-dinitrotoluene (DNT) introduce non-radiative deactivation pathways that compete with stimulated emission. We demonstrate that the induced cessation of the lasing action, and associated sensitivity enhancement, is most pronounced when films are pumped at intensities near their lasing threshold. The combined gains from amplifying materials and lasing promise to deliver sensors that can detect explosives with unparalleled sensitivity.
[structures: see text] An efficient synthesis of large iptycenes appended with alkoxy and ethynyl substituents is reported. The rigid shape-persistent iptycene scaffold prevents interactions between the polymer backbones and can be used to solubilize polymers containing less soluble but readily accessible comonomers to prepare functional, solution-processible poly(p-phenyleneethynylene) (PPE)-conjugated polymers. These polymers are highly emissive in thin films without significant excimer/exciplex formation as a result of the effective chain isolation enforced by the iptycene units.
A polymer blend system consisting of polystyrene grafted onto poly(p-phenylene ethynylene) (PS-g-PPE) and poly(styrene-block-isoprene-block-styrene) triblock copolymer (SIS) yields highly polarized emission due to the unidirectional alignment of the PPE mols. During the roll casting, the triblock copolymer microphase separates and creates unidirectionally aligned PS cylindrical microdomains in the rubbery PI matrix. PPE, a fluorescent conjugated polymer, was grafted with polystyrene (PS) side chains that enabled sequestration and alignment of these rigid backbone emitter mols. into the PS microdomains of the SIS triblock copolymer. Deforming the thermoplastic elastomer in a direction perpendicular to the orientation direction of the cylinders causes rotation of the PS cylinders and the PPE emitter mols. and affords tunable polarized emission due to reorientation of the PPE contg. PS cylinders as well as film thinning from Poisson effect. [on SciFinder(R)]
Blue poly(phenylene ethynylene) (PPE) electroluminescence (EL, see Figure) is obsd. in a hybrid org. light-emitting diode (OLED) with Commission Internationale de l'Eclairage coordinates of (x = 0.17; y = 0.20). Energy transfer from a hole-transport host to polystyrene-grafted PPEs is utilized to improve the poor LED characteristics of traditional PPE-based systems. [on SciFinder(R)]
A review. Poly(arylene ethynylene)s (PArEs) have been used in recent years as effective transducers for a variety of sensing purposes ranging from org. mols. such as Me viologen and TNT to biol. analytes. Their superior sensitivity to minor perturbations is fundamentally governed by the energy transport properties resulting from the extended conjugation of the polymer backbone. An understanding of the underlying principles of energy transport allows the design of sensors with greater sensitivity and specificity. Pioneering work with Me viologen as an electron-transfer quencher demonstrated that connecting receptors in series amplifies the sensing response compared to that of individual receptors. Since then, factors such as the electronic and structural nature of the polymers and their assembly architecture have proven to be important in improving sensory response. In this review, the authors present an overview of works to date by various groups in the field of PArE chemosensors and biosensors. [on SciFinder(R)]
Routine diagnostics and studies of Alzheimer's disease might benefit form the noninvasive optical imaging of amyloid-$\beta$ plaques in the brain. A rational design strategy for in vivo amyloid-imaging agents that enter the brain and selectively stain amyloid plaques is presented (see picture), and properties of a promising lead biomarker candidate are reported. [on SciFinder(R)]
2003
Polymers incorporating the triptycene subunit were prepared for the molecular-level design of low dielectric constant (low-kappa) materials that can be used to manufacture faster integrated circuits. Triptycenes having restricted rotation by multiple point attachment to the polymer backbone are shown to introduce free volume into the films, thereby lowering their dielectric constants. The triptycene containing polymers exhibit a number of desirable properties including low-water absorption and high thermal stability. Systematic studies wherein comparisons are made between two separate classes of triptycene polymers and their non-triptycene containing analogues demonstrate that proper insertion of triptycenes into a polymer backbone can give rise to a reduction in the material's dielectric constant while also improving its mechanical properties. These characteristics are desired by the semiconductor industry for the next generation of microprocessors and memory to provide insulation of the increasingly shrinking features.
A new approach based on a conjugated polymer/block copolymer guest/host system for the generation of polarized photoluminescence is reported. Synthetic modification of a poly(p-phenylene-ethynylene) (PPE) conjugated polymer is used for domain-specific incorporation into a cylindrical morphology block copolymer host matrix. Subsequent ordering of the host nanostructure via roll cast processing templates uniaxial alignment of the guest PPE. The ordered films are optically anisotropic displaying both polarized absorption with a dichroic ratio of 3.0 at 440 nm and polarized emission with a polarization ratio of 7.3 at 472 nm.
A canopy-shaped pyrrole derivative 2 was prepared, in which a sterically demanding pendant group is juxtaposed to the pyrrole fragment to minimize interstrand pi-pi stacking interactions in the resulting polymer. Anodic polymerization of 2 afforded highly conductive poly(2), the electronic structure of which was probed by various spectroelectrochemical techniques. A limited charge delocalization within poly(2) translates into a well-defined conductivity profile, properties important for resistivity-based sensing. Notably, the bulk conductivity was precisely modulated by a rapid and reversible deprotonation and reprotonation of the polymer backbone.
Indicators providing highly sensitive and functional group specific fluorescent response to diisopropyl fluorophosphate (DFP, a nerve gas (G-agent) simulant) are reported. Nonemissive indicator 2 reacts with DFP to give a cyclized compound 2+A- that shows a high emission due to its highly planar and rigid structure. Very weak emission was observed by the addition of HCl. Another indicator based on pyridyl naphthalene exhibits a large shift in its emission spectrum after reaction with DFP, which provides for quantitative ratiometric detection.
Segmented conducting polymers based upon a calix[4]arene scaffold are reported. The cone conformation creates a zigzag orientation of the polymer segments. Their acid-dependent conductivities are similar to the strong pH conductivity dependence of polyaniline which is said to be acid dopable. On the other hand, they have a segmented structure that imposes greater localization of the carriers. The conductivity of such a system can be considered to result from rapid self-exchange between discrete units. Hence, electron exchange between radical cations and p-diquinone salts produces the high conductivity of these polymers.
A review. Signal amplification for ultra-sensitive detection was achieved by energy migration in conjugated semiconducting polymeric assemblies. Crit. to optimizing this effect is the synthesis of non-aggregate polymers, the multi-dimensional directional transport of excited states (excitons), and extending the intrinsic excited state lifetime of conjugated polymers. We developed new water-sol. non-ionic conjugated polymers for use in biosensory applications, which can be used to provide highly sensitive/specific ultra-trace detection that is immune to specificity problems that plage ionic conjugated polymers. [on SciFinder(R)]
Ultrahigh mol. wt. polystyrene-b-polyisoprene block copolymers (BCs), noted for their photonic behavior, were imaged using transmission near-field scanning optical microscopy (NSOM) and NSOM polarimetry. The authors' improved scheme for polarization modulation (PM) polarimetry, which accounts for optical anisotropies of the NSOM aperture probe, enables mapping of the local di-attenuation and birefringence (with sep. aligned di-attenuating and fast axes) in these specimens with sub-diffraction limited resoln. PM-NSOM micrographs illuminate the mesoscopic optical nature of these BC specimens by resolving individual microphase domains and defect structures. [on SciFinder(R)]
The authors have introduced a new system for the detection of fluoride ion and successfully amplified the response using exciton migration in a semiconducting org. polymer. In contrast to most semiconductive-polymer sensor schemes that rely on changes in emission intensity, this sensory system uses a new fluorescence signal. The approach of directly interconnecting the indicator electronically with the polymer's band structure is a promising alternative to FRET schemes and the authors intend to apply this approach to other analytes of interest. [on SciFinder(R)]
A new nonionic water-sol. fluorescent conjugated poly(phenylene ethynylene) is reported with hydroxyl and amide side chains surrounding an arom. polymer backbone. [on SciFinder(R)]
Lasing conditions in a dye-doped cholesteric liq. crystal (ChLC) were studied in view of optical modes for the light propagating in ChLCs using a polymeric dye with the transition dipole moment parallel to the local director of the ChLC host. Lasing always occurs at the lower-energy edge of the photonic gap. This is because that the optical eigen mode at the lower-energy gap is linearly polarized parallel to the director, while it is perpendicular at the higher-energy gap. Because of this well-defined lasing condition, low-threshold lasing was successfully achieved. [on SciFinder(R)]
The synthesis, spectroscopy, and electrochem. is presented of bis[oligo(thiophene)] monomers where the sterics of covalently attached subunits enforced oblique spatial orientations. The synthetic scheme applied to a variety of chromophores; std. bromination and cross-coupling chem. afforded bis(terthienyl)benzene systems in high yield. Model systems were prepd. consisting of mono(terthiophene)s to probe the effects that van der Waals contacts impose during electropolymn. and methyl-blocked analogs to examine the electrochem. properties of derivs. that do not undergo polymn. The extent of delocalization between chromophores as deduced from electrochem. studies is discussed and viable electrochromic polymers are demonstrated. [on SciFinder(R)]
2004
The authors studied the effects of an anisotropic nematic liq, crystal (NLC) defect layer introduced between polymeric cholesteric liq. crystals (PCLC) layers. It was found that the modulation of reflectance exceeds the 50 % limitation provided by simple CLC photonic bandgap (PBGs) due to the birefringence of the defect layer. Moreover, by adjusting the PBG region to be coincident with the fluorescent emission band for the guest polymer dye, sharp defect-mode lasing was obsd. successfully at the defect mode in the middle of PBG. From these results, it was demonstrated that the configuration of a PCLC with a polymer-dye-doped anisotropic defect layer is very attractive for lasing. [on SciFinder(R)]
Decreased spectral overlap between a donor biotinylated poly(p-phenylene ethynylene) and a chromophore-labeled streptavidin acceptor leads to better observed fluorescence resonance energy transfer.
A fluorescent poly(phenylene ethynylene) containing calix[4]arene-based receptor units has a sensitivity to quenching by the N-methylquinolinium ion that is over three times larger than that seen in a control polymer lacking calix[4]arenes.
The orientational phase diagram and ordering of guest liq. cryst. (LC) rods in a host liq. cryst. polymer (LCP) matrix quenched below the glass transition is detd. by field theory. Microscopic anisotropic interactions can align the LC rods to each other and also align LCP matrix side chains and the LC rods in the plane normal to the local LCP chain contour. The authors' numerical anal. suggest ways to exploit host entropy, anisotropy of microscopic interactions and manipulate properties of LC rods for modern applications. The authors predict a nematic-nematic discontinuous orientational transition from a guest stabilized to a guest-host stabilized region and a reentrant transition from a guest stabilized nematic region to a host only stabilized regime. A detailed anal. of phase boundaries transitions and ordering is presented. [on SciFinder(R)]
Lasing in dye-doped chiral nematic liq. crystals (N*LCs) was studied. To demonstrate the advantages of using a polymer dye, that is highly aligned along the local director of N*LCs, over com. small mol. dyes such as 4-(dicyanomethylene)-2-Me-6-(4-dimethylaminostryl)-4H-pyran (DCM), comparative studies of the fluorescence, lasing conditions and order parameters were made using polymer-dye doped N*LC and DCM-doped N*LC cells. Right- and polarized fluorescence spectra for both dyes were accurately simulated by taking account of their d. of modes and order parameters. The greater alignment afforded by the polymer dye in N*LCs provides ideal conditions for lasing. [on SciFinder(R)]
Many pathogens that infect humans use cell surface carbohydrates as receptors to facilitate cell-cell adhesion. The hallmark of these interactions is their multivalency, or the simultaneous occurrence of multiple interactions. We have used a carbohydrate-functionalized fluorescent polymer, which displays many carbohydrate ligands on a single polymer chain, to allow for multivalent detection of pathogens. Incubation of a mannose-functionalized polymer with Escherichia coli yields brightly fluorescent aggregates of bacteria. These results show that carbohydrate-functionalized fluorescent polymers are a versatile detection method for bacteria. Future design of detectors for other pathogens only requires information on the carbohydrates bound by the organisms, which has been exhaustively reported in the literature.
Dynamic nuclear polarization (DNP) experiments in rotating solids have been performed for the first time using biradicals rather than monomeric paramagnetic centers as polarizing agents. Specifically, two TEMPO radicals were tethered with a poly(ethylene glycol) chain of variable length where the number of glycol units was 2, 3, or 4. NMR experiments show that the signal observed in DNP experiments is approximately inversely proportional to the length of the chain. Thus, the shorter chain with larger electron dipolar couplings yields larger enhancements. The size of the enhancement is a factor of 4 larger than obtained with the identical concentration of monomeric nitroxide radicals achieving a value of approximately 175 for the n = 2 chain.
The prepn. of two highly emissive conjugated polyacetylenes with tethered rotaxane repeat units are reported. Hydrogen bonding between acidic alcs. and the N-heteroarom. groups in the rotaxanes attenuates polymer fluorescence. In addn., the rotaxane groups create precise three-dimensional pockets for metal binding, which results in fluorescence quenching. Exposing thin films of Zn-doped polymers to alc. vapors reverses the quenching by up to 25%. [on SciFinder(R)]
New polymers having high solid-state fluorescence quantum yields and the ability to tune their electron affinity without effecting their band gap using hyperconjugative interactions is reported. The novel three-dimensional poly(phenylene vinylenes) having [2.2.2] bicyclic ring systems shown were synthesized, and the different hyperconjugative perturbations provide differential fluorescence sensory quenching responses to electron-rich and electron-deficient analytes in solution and solid thin films.
Prepn. and characterization of 3 high mol. wt. iptycene-contg. polybutadienes as well as their starting monomers (derived from anthracene, tetracene, and 1,4-anthraquinone via initial Diels-Alder reactions followed by operations such as hydrogenation, dehydrohalogenation, and dehydration) are described. All monomers at least possessed a butadiene unit that was used for radical polymn. leading to polymers presenting an internal free vol. that could be utilized for the enhancement of miscibility and redn. of phase sepn. in polymer blends. [on SciFinder(R)]
New thermally responsive fluorescent polymers conjugated with poly(N-isopropylacrylamide) (polyNIPA) were synthesized. A nonionic water-sol. poly(phenyleneethynylene) (PPE) was end-capped with a di-tert-butylnitroxide deriv., and subsequent nitroxide-mediated radical polymn. of NIPA afforded PPE-polyNIPA block copolymers. These copolymers phase-sep. from aq. solns. upon heating, and the resultant ppts. are efficiently collected by filtration. The fluorescent spectra of the ppts. indicate the absence of strong assocns. between the PPE $π$-systems. Furthermore, the fluorescent intensities of the collected solids have a linear correlation with the polymer concns. in the solns. of origin. When copolymers are thermally copptd. with dye-labeled (rhodamine B) polyNIPA materials, the dye is localized to the PPE segments, inducing fluorescent resonance energy transfer from the PPE segment (donor) to the dye (acceptor). [on SciFinder(R)]
Novel half-disk mesogens of general structure 6,7,10,11-tetrakis(alkoxy)triphenylene-1,4-dione were synthesized and their phases characterized by polarized microscopy, DSC and x-ray diffraction. These compds. form hexagonal columnar mesophases upon heating, despite their half-disk mol. shapes. X-ray diffraction suggests that within the mesophases a dimeric mesogenic subunit exists, driven by dipolar forces, in which the mols. are oriented antiparallel to one another. Such a dimer is approx. disk-shaped, and may explain the formation of columnar phases by these half-disk mols. [on SciFinder(R)]
Several $\beta$-diketonate ligands were synthesized which have a chiral directing element and/or a polymerizable moiety. Octahedral iron complexes of these ligands were crosslinked in the columnar hexagonal phase using acyclic diene metathesis (ADMET) polymn. The resulting liq. crystal materials were chiral and retained the order of the mesophase. [on SciFinder(R)]
A review of the amplifying ability of semiconductive org. polymers in sensory schemes and its use for the detection of nitroarom. explosives. Semiconductive org. polymers serve as extremely efficient conduits for the transport of optically induced excitations and it is this transport property that allows for the high sensitivity of these materials to trinitrotoluene (TNT) and dinitrotoluene (DNT), the primary explosives used in landmines. Systematic mol. designs for the formation of improved sensitivity sensory materials are described. [on SciFinder(R)]
The lasing characteristics were studied of a dye-doped nematic layer sandwiched by 2 polymeric cholesteric liq. crystal (CLC) films as photonic band gap (PBG) materials. The nematic layer acts as a defect layer, the anisotropy of which brings about the following remarkable optical characteristics: (1) reflectance in the PBG region exceeds 50% due to the retardation effect, being unpredictable from a single CLC film; (2) efficient lasing occurs either at the defect mode wavelength or at the photonic band edge; and (3) the lasing emission due to both the defect mode and the photonic band edge mode contains both right- and left-circular polarizations, while the lasing emission from a dye-doped single CLC layer with a left-handed helix is left-circularly polarized. [on SciFinder(R)]
A review. Synthetic strategies to control interchain electronic communications within conjugated polymers (CPs) are described. Novel chem. architectures built on iptycenes, metallorotaxanes, and canopied pyrroles restrict the dimensionality of electronic structures responsible for exciton and charge transport. Structure-property relationships emerging from studies of selected systems are discussed, focusing on their implications for the sensitivity of these materials as sensors. [on SciFinder(R)]
An efficient synthetic route to $\beta$-linked dipyrrole monomers has been developed. Electrochem. polymn. of these monomers leads to the incorporation of polycyclic arom. residues into a polymer backbone. The resulting conjugated polymer films are electroactive, robust electrochromic materials that are highly delocalized in their oxidized forms. [on SciFinder(R)]
We study the phase diagram and orientational ordering of guest liquid crystalline (LC) rods immersed in a quenched host made of a liquid crystalline polymer (LCP) matrix with mobile side chains. The LCP matrix lies below the glass transition of the polymer backbone. The side chains are mobile and can align to the guest rod molecules in a plane normal to the local LCP chain contour. A field theoretic formulation for this system is proposed and the effects of the LCP matrix on LC ordering are determined numerically. We obtain simple analytical equations for the nematic/isotropic phase diagram boundaries. Our calculation show a nematic-nematic (N/N) first order transition from a guest stabilized to a guest-host stabilized region and the possibility of a reentrant transition from a guest stabilized nematic region to a host only stabilized regime separated by an isotropic phase. A detailed study of thermodynamic variables and interactions on orientational ordering and phases is carried out and the relevance of our predictions to experiments and computer simulations is presented.
2001
We have investigated a model thin film sensory system in which analytes diffuse into multilayers of a fluorescent conjugated polymer. The film thickness is precisely controlled by depositing discrete monolayers by the Langmuir-Blodgett technique. The effects of analyte mass transport and energy migration on the photophys. properties of the films were investigated by conducting UV-vis, fluorescence, and elec. cond. measurements. Thin films show different properties when compared to relatively thick films due to prevailing surface phenomena. The diffusion const. of the analyte through the films is estd. to be ∼7 × 10-14 cm2/s from an anal. of a phenomenol. model. A bilayer LB film exposed to the analyte implies higher sensitivity in fluorescence quenching compared to a soln. system due to a fast interpolymer energy migration in the condensed phase. However, as the no. of layers increases, the efficiency of fluorescence quenching decreases. The difference between a sensory system with emissive surface traps and one with bulk distributed quenching traps is discussed. [on SciFinder(R)]
Regiospecific substituted polyanilines were prepd. via electropolymn. of methoxy-substituted dimeric and trimeric oligoanilines. The oligoaniline monomers were synthesized utilizing Pd-catalyzed aryl amination cross-coupling chem. The single-crystal x-ray structure of one of the oligomers is presented. The oligoaniline monomers were electropolymd. in 1 M H2SO4, and the electrochem. behavior and potential-dependent in situ cond. of the regiospecific polyaniline films was compared to that of random copolymers obtained from solns. of aniline and o-anisidine of the same molar ratio. The regiospecific polyanilines exhibited higher cond., which may be attributed to a more cryst. and regular structure. Differences in the oxidn. potential of the polymers are obsd. depending on the degree of methoxy substitution. [on SciFinder(R)]
The role of conjugated polymers in emerging electronic, sensor and display technologies is rapidly expanding. In spite of extensive investigations, the intrinsic spectroscopic properties of conjugated polymers in precise conformational and spatial arrangements have remained elusive. The difficulties of obtaining such information are endemic to polymers, which often resist assembly into single crystals or organized structures owing to entropic and polydispersity considerations. Here we show that the conformation of individual polymers and interpolymer interactions in conjugated polymers can be controlled through the use of designed surfactant poly(p-phenylene-ethynylene) Langmuir films. We show that by mechanically inducing reversible conformational changes of these Langmuir monolayers, we can obtain the precise interrelationship of the intrinsic optical properties of a conjugated polymer and a single chain's conformation and/or interpolymer interactions. This method for controlling the structure of conjugated polymers and establishing their intrinsic spectroscopic properties should permit a more comprehensive understanding of fluorescent conjugated materials.
[structure: see text]. New poly(phenylene ethynylene)s (PPEs) and poly(phenylene vinylene)s (PPVs) that are highly emissive in solution and thin films were prepared utilizing palladium-catalyzed cross-coupling between new 1,4-diiodo-2,3-dialkoxybenzene- and iptycene-containing monomers. The absorption and emission spectra of the resulting polymers consistently showed a significant blue shift relative to the corresponding polymer analogues containing 2,5-dialkoxyphenylenes.[on SciFinder (R)]
Recent research on producing polyiptycenes (polymers contg. triptycene units in the backbone) is discussed. The highly rigid structure of these polymers provides large degrees of free vol. and a host of novel properties. [on SciFinder(R)]
To achieve highly stable electrochromic materials, 4,5-bis(2-/3-thiophenyl)-1,2-dialkoxybenzene precursors were prepd., cyclized (aromatized), and the resulting naphthodithiophene monomers were subjected to oxidative polymn. (chem. as well as electrochem.). The precursors, monomers, and polymers were characterized by spectroscopic and electrochem. methods. [on SciFinder(R)]
Two triptycene derivs. were incorporated into a polyvinyl chloride (PVC) film or into the nematic phase of 4-(trans-4-pentylcyclohexyl)benzonitrile. The supramol. alignment taking place was visualized by polarized UV-vis and IR spectroscopy. The director axis of the nematic liq. crystal or the stretching direction of the polymer film are parallel to the the long axis of the guest mol. The degree of alignment for the 2 triptycene derivs. was detd. via the aspect ratio in comparison to anthracene. The alignment provided by the uniaxial PVC film was lower than that for the LC. A threading mechanism was proposed for the supramol. arrangement based on the minimization of the free vol. [on SciFinder(R)]
A review, with 28 refs., on the development of polymers as 1D photonic crystals with focus on self-assembled block copolymers. The use of plasticizer and homopolymer blends of diblock copolymers to increase periodicity and the role of self-assembly in producing 2D and 3D photonic crystals are discussed. The use of inorg. nanoparticles to increase the dielec. contrast and of a biasing field during self-assembly to control the long-range domain order and orientation are outlined. In-situ tunable materials fabricated via a mechanochromic materials system are also described. The inherent optical anisotropy of extruded polymer films and side-chain liq.-cryst. polymers provides flexibility for novel optical designs. [on SciFinder(R)]
A highly selective allosteric fluoride recognition system is described consisting of a doubly strapped porphyrin (I) that contains 2 small hydrogen-bonding cavities not being able to bind larger anions. Compd. I was prepd. by condensation of strap moieties with a $\alpha$$\beta$$\alpha$$\beta$-tetrakis(2-aminophenyl)porphyrin under high diln. conditions. By comparison with related strapped porphyrins contg. 4 linkers the cofacial distance between the phenyls in the straps and the porphyrin was estd. to be 3-4 \AA creating a small pocket suitable for F- binding but excluding larger anions. The proximate location of the strap moieties to the porphyrin in I was confirmed by NMR spectroscopy in CDCl3. The Soret band of I in dichloromethane was split into 2 bands of equal intensity indicating that the strap presented a significant perturbation caused by $π$-$π$ interactions of the bis(dithienyl)-benzene moieties with the porphyrin. Upon addn. of F- to a soln. of I in DMSO the split Soret band shifted to a single band with a simultaneous red shift of the Q band whereas no absorbance changes were obsd. upon exposure of larger anions including Cl-, Br-, J-, CN-, and H2PO3-. The plots of absorbance changes vs. TBAF showed a sigmoidal curve indicating that the F- binding to I is cooperative. This binding was analyzed with the Hill equation and further characterized using Scatchard plots. Given the obsd. 1:2 binding stoichiometry energy-minimized geometries were computed suggesting that the cavities in I are contracted. The calcd. structure with 2 bound F- caused an expansion of the cavity that sepd. the planes of the porphyrin and the straps by 4.9 \AA. From studies of the influence of a second coexisting halide ion on the UV/Vis spectrum of the I(F2-) complex it was estd. that the affinity of I to F- was ∼104 times higher than for other halides. A conducting polymer based upon I displays both electrochem. and cond. responses to F- and no response to Cl-. [on SciFinder(R)]
Two new bimetallic Pd complexes I (R = t-Bu, Ph) have been synthesized and characterized and their catalytic activity checked for the aldol reaction of aldehydes with Me isocyanoacetate. Each palladium atom is coordinated to an SCS-type ligand and the two pincer units are linked by a chiral spacer. The catalytic aldol reaction of Me isocyanoacetate with aldehydes proceeds quickly but no significant diastereoselectivity and enantioselectivity is found. The comparison with a homologous mononuclear Pd complex shows no differences with the bimetallic compds., concluding that there is no cooperativity between the metal centers. Two silica-supported catalysts prepd. with a bimetallic compd. show catalytic activity with very minor enantioselectivity. [on SciFinder(R)]
2002
A series of host-guest complexes of tungsten(VI)-oxo calixarenes substituted with two bithiophene groups at the upper rim were prepd. The bithienyl tungsten(VI)-oxo calixarenes were prepd. by Stille coupling of the complex with 2-tributylstannyl bithiophene and hydrolysis of the glycol ligand yielding the monomer precursor as the DMSO adduct. Upon removal of DMSO, the complex readily reacted with substituted formamides to produce the host-guest adduct monomers. These adducts undergo electrochem. polymn. that results in conducting polymers that contain guest mols. inside the calixarene cavity. The cond. of the polymers is dependent on the nature of the guest, being significantly lower for the disubstituted formamide complexes. [on SciFinder(R)]
A sensor for nitric oxide is reported that uses a novel redox matching mechanism to induce a resistance change upon binding this important ligand to cobalt. [on SciFinder(R)]. This work was made possible by a Postdoctoral Fellowship from the Japan Society for the Promotion of Science to T. Shioya and the generous financial support of the Office of Naval Research.
The synthesis and characterization of various thiophene-based bent-rod liq. crystals are reported, and the effects of varying lateral dipole and core desymmetrization upon mesophase behavior are described. Incorporation of desymmetrized core 7 into the mol. framework has very different consequences depending upon whether n-alkoxy or tetracatenar-type end groups were used. Tetracatenar-type mesogens 8-11 are significantly less mesogenic than the previously reported sym. series 3. When sym. straight-chain compds. 13-17 and unsym. straight-chain compds. 18-21 were studied, however, the desymmetrized core gave rise to mesophases with much broader temp. ranges. Variable temp. x-ray diffraction of these compds. suggests the formation of antiparallel dimers of mols. within the liq. crystal phase, and this may explain the relatively stable mesophases formed by these compds. and their incompatibility with chiral induction. The effects of altering the lateral substituents were also explored, and 3,4-difluorothiophene-based compds. 24-27 exhibit broad nematic mesophases. [on SciFinder(R)]
A new class of nematic liq. crystals with triptycenes built into a p-dialkoxybis(phenylethynyl)benzene mesomorphic core were prepd. Triptycenes are appended on the center or terminal ring of the mesogen, leading to sym. liq. crystals and reduced symmetry liq. crystals, resp. Both types displayed monotropic behavior, with the asym. compds. having unusual phase behavior, lacking distinct crystn. transitions, and forming a glassy mesophase. A chiral analog is nonmesomorphic, but induced chiral nematic phases when doped into achiral triptycene-contg. analogs. Rotation is phys. hindered normal to the director and, hence, this new liq. crystal architecture may allow for the synthesis of a single component liq. crystal that displays a biaxial nematic phase. [on SciFinder(R)]
Several pendant arenes, e.g. I (R = Me, MeO), were prepd. using a variety of Pd-catalyzed cross-coupling techniques. Under strongly acidic conditions, these arenes underwent double annulations to afford the corresponding polycyclic aroms., e.g. II, in good to excellent yields. The scope of this chem. also includes heteroarom. and heteroat. substituted bis(arylethynyl) arenes thus providing an electronically diverse array of extended arom. systems. [on SciFinder(R)]
A range of mesogenic mols. varying in both bend angle and strength of lateral dipole were synthesized, and their phase behavior was characterized by polarizing microscopy, thermal anal., x-ray diffraction, and electrooptical measurements. The authors find the general destabilization of the liq. crystallinity caused by strong lateral dipolar groups and the bent mol. shape are off-set in mesomorphic tetracatenars, which display stable nematic, smectic, columnar, and cubic mesophases. The broad mesomorphism of the tetracatenars contg. lateral dipoles and their incompatibility with chiral induction are explained by considering that loosely correlated dimers exist within the mesophases. Chiral mesophases of derivs. with strong lateral dipoles were achieved by attaching fewer or different side chains to each end of the mesogen. [on SciFinder(R)]
Fluorescent poly(phenylene vinylene)s and poly(phenylene ethynylene)s contg. rigid triptycene groups were prepd. using Suzuki and Sonogashira protocols. The triptycene groups impart extraordinary soly. to conjugated polymers even in the absence of flexible side chains; addnl. t-Bu groups were introduced, which were found to further enhance soly. Stable solns. of poly(phenylene vinylene)s and poly(phenylene ethynylene)s in nematic liq. crystal 1-(trans-4-hexylcyclohexyl)-4-isothiocyanatobenzene (6CHBT
We present the relationship between the spatial arrangement and the photophys. properties of fluorescent polymers in thin films with controlled structures. Eight surfactant poly(p-phenyleneethynylene)s were designed and studied. These detailed studies of the behavior of the polymers at the air-water interface, and of the photophys. properties of their transferred LB films, revealed key structure-property relationships. Some of the polymers displayed $π$-aggregates that are characteristic of an edge-on structure at the air-water interface. Monolayer LB films of these polymers showed greatly reduced quantum yields relative to soln. values. Other polymers exhibited a highly emissive face-on structure at the air-water interface, and did not form $π$-aggregates. The combination of pressure-area isotherms and the surface pressure dependent in situ UV-vis spectra of the polymers at the air-water interface revealed different behavioral details. In addn., the UV-vis spectra, fluorescence spectra, and quantum yields of the LB films provide design principles for making highly emissive films. [on SciFinder(R)]
We present a general strategy for obtaining large sulfur-contg. polycyclic aroms. from thienyl precursors through iron(III) chloride mediated oxidative cyclizations. By placing thienyl moieties in close proximity to adjacent arenes, we have directed the oxidized intermediates into controlled cyclization pathways, effectively suppressing polymer formation. Utilizing these cyclized compds. and their thienyl precursors, we have studied cyclization/polymn. pathways of polymers such as poly(2). The unsubstituted positions $\alpha$ to the sulfur atoms within these arom. cores allowed for efficient halogenation and further functionalization. As a demonstration, we prepd. a series of arylene-ethynylene polymers with varying degrees of chromophore aromatization and used them to probe the effects of synthetically imposed rigidity on polymer photophys. behavior. The symmetries and effective conjugation pathways within the monomers play a key role in detg. photophys. properties. We obsd. that rigid, aromatized chromophores generally led to increased excited-state lifetimes by decreasing radiative rates of fluorescence decay. [on SciFinder(R)]
Triptycenes have general applicability for increasing the alignment of fluorescent and dichroic dyes in LC hosts. Dyes contg. varying nos. of triptycenes were synthesized to study the effect of free-vol. alignment of triptycenes on the alignment of dyes. These dyes were designed such that multiple triptycenes could be incorporated and the triptycene-free vol. is coincident to the aspect ratio of the dye, allowing a cooperative effect to increase their overall av. alignment. With increasing triptycene incorporation, a stepwise increase in the alignment parameters of each dye was seen. It was also found that the attachment of one triptycene group has a negligible effect on the optical switching response times of the dyes. This can be a powerful tool for designing dyes with higher alignments for a variety of applications including guest-host reflective LCDs and holog. data storage. [on SciFinder(R)]
A liq. cryst. vanadyl complex was studied by DSC, polarizing optical microscopy, the reversal current technique, x-ray diffraction and frequency domain dielec. spectroscopy. The compd. exhibits three columnar phases: rectangular ordered (Colro), rectangular disordered (Colrd), and hexagonal disordered (Colhd), all of which show a dielec. relaxation process at low frequencies. In the Colro low temp. phase this process seems to be connected with a slow relaxation of polarized polymeric chains inside the columns (mHz frequency range). However, in the Colhd high temp. disordered phase this relaxation is faster (Hz range). It is interesting that the liq. cryst. phases studied show enhanced cond. which changes by four orders of magnitude from 10-9 S m-1 in the orientationally disordered crystal (an ODIC phase) to 10-5 S m-1 in the Colhd high temp. phase. Such a value of the cond. is typical for semiconducting materials. [on SciFinder(R)]
A novel approach to the fabrication of poly(p-phenylene ethynylene) (PPE) brushes on oxidized silicon surfaces using ring-opening metathesis polymn. was demonstrated. Chem. bound 71-110 \AA thick PPE brushes were constrained from forming aggregates and gave higher emission quantum yields than spin-cast films. [on SciFinder(R)]
The ability of excited states (excitons) to migrate rapidly and efficiently through conjugated polymers makes these materials ideal for use in sensors based on fluorescence quenching or amplification of fluorescence signals. The structural features of the conducting polymers allow for design of highly sensitive fluorescent sensors for specific analytes such as the explosive trinitrotoluene (TNT) and to create assemblies that control energy transfer along a predetd. path. The principles involved have broad utility in the design of sensory materials and of electronic devices and display components based on electronic polymers. [on SciFinder(R)]
In an contribution to an article in the same issue, the advantages of the rotaxane approach (precise mol. insulation technique) in the design of polymer light-emitting devices are highlighted. Semiconducting polymers are threaded through cyclodextrins and are capable of both emitting light and transporting charge. [on SciFinder(R)]
1999
The photophys. and energy transport properties of poly(p-phenylene ethynylene) were studied in thin films. Highly aligned films of a precise thickness, prepd. by sequential monolayer deposition using the Langmuir-Blodgett technique, were surface modified with luminescent traps (Acridine Orange, AO) for energy transfer studies. The degree of energy transfer to the traps was studied as a function of the AO concn. and the no. of polymer layers. An increased efficiency of energy transfer to the traps was obsd. with increasing nos. of layers to an approx. thickness of 16 layers. This behavior is consistent with a transition to a three-dimensional energy migration topol. A phenomenol. model for the transport was proposed, and solns. were obtained by numerical methods. The model yields a fast (>6 × 1011 s-1) rate of energy transfer between polymer layers and a diffusion length of more than 100 \AA in the Z direction (normal to the film surface). [on SciFinder(R)]
Langmuir-Blodgett (LB) mol. processing of conjugated polymers [poly(phenylene ethynylenes)] into highly aligned films has revealed conditions for the formation of liq. cryst. monolayer films that structurally evolve into fibril aggregates. The structural requirements for poly(phenylene ethynylene)s to display liq. cryst. phases capable or alignment by LB methods were detd. The reconstruction of monolayers into fibril structures requires a low glass-transition temp. (Tg), weak surface anchoring, and a monolayer with a high energy that can be stabilized by reorganization. This assembly of polymers into aggregated structures produces rigid structural units analogous to naturally occurring fibrous proteins such as collagen and elastin. These oriented, shape-persistent nanoscale structures create new possibilities for the construction of complex supramol. structures, and this capability was demonstrated by the formation of a nanoscale grid. [on SciFinder(R)]
2,4,6-Tris[p-(p'-n-alkylphenyliminomethylene)phenoxy]-s-triazines (3) are calamitic liq. crystals based on x-ray diffraction patterns, optical textures, and mol. modeling results. Replacement of the Schiff's base moieties in the mesogenic arms to form 2,4,6-tris(p-n-octyloxycarbonylphenoxy)-s-triazine (7) did not result in a liq. cryst. compd. The tricarbonate 2,4,6-tris(p-cholesteryloxycarbonyloxyphenoxy)-s-triazine (11) is liq. cryst. based on the optical textures obsd., although the mesophase type could not be detd. due to the high melting transition and thermal instability of this compd. The use of six ester groups around the triazine nucleus, as 2,4,6-tris(3,5-dicarboalkoxyphenoxy)-s-triazines (13), resulted in compds. which displayed normal melting behavior and no detectable mesomorphism. [on SciFinder(R)]
A review with 11 refs. is given on the authors' work with emphasis on fluorescence-based sensor schemes and new chemoresistive transducer mechanisms. [on SciFinder(R)]
A review with 356 refs. on the wide variety of transition metal-centered conjugated/conducting polymers and networks. Organometallic-based systems, such as organometallic acetylenic polymers, metallacycle polymers, polyferrocenylenes, transition metal aryl complexes, pendant organometallic polymers, pendant ferrocene polymers, organometallic coordination polymers, as well as various types of coordination complexes, such as one-dimensional and two-dimensional phthalocyanine polymers, conjugated porphyrin polymers, polymers derived from schiff-base complexes, polymeric bipyridine and related complexes, and diisocyanoaryl ligands are described. [on SciFinder(R)]
Results are presented of luminescence (PL) and electroluminescence (EL) studies on color variable LEDs based on parahexaphenyl (PHP) and polypyridine (PPy) related polymers and copolymers. In the fabricated bilayer and 3 layer heterostructure EL devices, PPy and poly(pyridyl vinylene phenylene vinylene) (PPyVPV) are used as electron and hole transport layers resp. For ITO/PPyVPV/PHP/PPy/Al EL device, multi-colors emission in the wide range of the visible spectrum from blue (425 nm) to green (530 nm) and near IR (700 nm) were obtained. The optical and elec. characteristics of bilayer and multilayer EL devices are presented and discussed. [on SciFinder(R)]
The authors report the fabrication and study of color variable multilayer light emitting devices based on pyridine-contg. conjugated polymers and para-sexiphenyl (6P) oligomer. Polarity controlled two color devices were fabricated by sandwiching the emitting layer in between emeraldine base and sulfonated forms of polyaniline (SPAN). The emitting layer typically is a blend of two polymers, one of which is a pyridine-based copolymer (PPyVPV). The devices can be operated under either polarity of driving voltage with different colors of light being emitted from different locations. Under forward bias, red light is generated from PPyVPV/SPAN interface. Under reverse bias, light is generated from the bulk of the emitting layer whose color is dependent on the materials used. Voltage controlled multicolor devices were fabricated by combining the pyridine-based polymers with the 6P oligomer. Voltage dependent multicolor emission was obsd. in both bilayer and trilayer devices. The emission colors of single devices cover a wide range of visible spectra whose CIE color coordinates vary from blue to white to green with increasing voltages. [on SciFinder(R)]
Voltage- and polarity-controlled multilayer multicolor light-emitting test devices based on pyridine-contg. conjugated polymers and derivs. of polyacetylene were fabricated and evaluated. The polymer blends of poly(pyridyl vinylene deriv.) and poly(bis(hexadecyloxy)phenylene-vinylene) and poly(di-Ph Bu acetylene) or poly(hexyl Ph acetylene) are used as the emitting material. Sulfonated poly(o-methoxyaniline) is the redox layer and ITO and Al were used as electrodes. The devices emit red light under forward bias and multiple colors of light (from orange-red to green) under reverse bias. The colors under reverse bias are controlled by the magnitude of the applied voltages. [on SciFinder(R)]
A no. of bent-rod mols. of thiophene derivs. were prepd., and their phase behavior was studied. Mostly these compds. have the nematic phase. The crystal structure of 3,4-dicyano-2,5-bis[(methoxyphenyl)ethynyl]thiophene shows that the antiparallel arrangements of lateral dipoles seems most likely. Crystals are monoclinic, space group P21/n, with a 11.5757(2), b 10.03300(10), c 17.3438(4) \AA, $\beta$ 100.4580(10)°; Z =
Square antiprism Zr tetrakis-$\beta$-diketonate complexes with 24 alkoxy chains organize in columnar liq. crystal phases. X-ray diffraction and polarized microscopy studies on complexes with n-alkoxy side chains revealed a columnar hexagonal phase. These sandwich-shaped compds. have much lower transition temps. than their discotic analogs, which leads to the desirable attribute of room temp. liq. crystallinity. The addn. of two branching Me groups to the alkoxy chains dramatically alters the properties of these materials. The branched side chain analogs exhibited a higher clearing point while the liq. crystallinity is maintained at room temp. The branching Me groups also induced a bulk reorganization of the material to a rare columnar oblique phase (Colob). [on SciFinder(R)]
A series of thiophene-appended RuII(bpy)3 derivs., Ru(1)3, Ru(2)3, Ru(3)3, Ru(bpy)2(1), Ru(bpy)2(2), and their resulting polymers were synthesized and characterized. The bpy ligands 5,5'-bis(5-(2,2'-bithienyl))-2,2'-bipyridine (1), 4,4'-bis(5-(2,2'-bithienyl))-2,2'-bipyridine (2), and 4-(5-(2,2'-bithienyl))-2,2'-bipyridine (3), all contain electrochem. polymerizable bithienyl moieties. The monomers Ru(2)3, Ru(3)3, Ru(bpy)2(1) and Ru(bpy)2(2) display spectroscopic features that are similar to the ligand-based and MLCT [metal-to-ligand charge-transfer] bands found for Ru(bpy)3. The cyclic voltammograms of all of these polymers display both metal-centered and thiophene-based electroactivity. High redox cond. was found in poly(Ru(2)3) and poly(Ru(3)3) for both the thiophene-based oxidn. and metal-based redn. processes. These results indicate that the polymers display charge localization for both the metal complexes and the tetrathienyl connecting units. The degree of interconnection (no. of linkages) and the substitution pattern were found to control the cond. of these polymers. The highest cond. (3.3 × 10-3 S cm-1) was found for poly(Ru(2)3), which is able to have up to 6 linkages with other ruthenium complexes and possessing a 4,4'-substitution pattern that allows effective orbital overlap of the conjugated polymer backbone with the ruthenium centers. [on SciFinder(R)]
O-Ethynylphenylcarbonyl compds. undergo cyclization to 2-benzopyrylium salts on treatment with acid. Treatment of these compds. with NH3 gave isoquinolines. 1-Ethoxy-2-benzopyrylium salts were partially hydrolyzed to the hydroxy analogs and 1-dimethylaminoisoquinolines were accompanied by the amino analogs. [on SciFinder(R)]
The synthesis, electrochem., and spectroscopic behavior of tetradentate bis(salicylidenimine) transition metal complexes are reported. Appending these complexes with 3,4-ethylenedioxythiophene (EDOT) moieties allows for electrochem. polymn. at much lower potentials than the parent SALEN complexes. The resulting polymers display well-defined org.-based electrochem. at potentials <0.5 V vs. Fc/Fc+. The EDOT-modified N,N'-ethylene bis(salicylidene), N,N'-o-phenylene bis(salicylidene), and N,N'-trans-cyclohexylene bis(salicylidene) complexes I and II, III and IV, and V and VI, resp., display cyclic voltammograms with four org.-based redox waves. Increasing the interchain sepn. through the use of nonplanar bis(salicylidene) ligands results in only two redox waves. The cond. of the copper-based polymers decreases with increasing interchain spacing, with the max. cond. being 92 S cm-1 for poly(I) and 16 S cm-1 for the stilbenediamine complex polymer. The nickel complexes were less sensitive to increased interchain sepn. and showed cond. greater than 48 S cm-1 regardless of interchain spacing and near 100 S cm-1 in the case of poly(IV). In situ spectroelectrochem. was consistent with the segmented electronic nature of these polymers. Cyclic voltammetry of an analogous uranyl complex revealed that two electrons per repeat unit were removed during oxidn. From electrochem. and in situ EPR spectroscopic studies suggest that $π$-aggregation processes take place in those polymers in which close interchain spacing is allowed. [on SciFinder(R)]
Liq. crystals displaying a Colh (columnar hexagonal) phase can display a cooperative chiral state. In the authors model the hexagonal symmetry is an integral feature that favors the chiral state. This latter point is also supported by the recent work on fluxional 8 vertex Zr4+ mesogens (Trazaska et al. 1999) with the same ligands as used in this paper, wherein no increase was found in the CD signal on cooling from the isotropic phase into the oblique columnar phase. [on SciFinder(R)]
2000
A new transduction mechanism based on the aggregation of conjugated sensory polymers induced by K+ ions is reported; this new system displays enhanced sensitivity because of energy migration processes and has a high selectivity for K+ over Na+ ions. The poly(p-phenylene ethynylene)s were synthesized by the Sonogashira-Hagihara coupling reaction. [on SciFinder(R)]
A highly electroactive polythiophene-metallophthalocyanine hybrid material was prepd. which exhibits cond. more than three orders of magnitude higher than that of know macrocycle-connected polymers. The triptycene-contg. phthalocyanine macrocycle monomers have electrochem. polymerizable thiophene moieties on alternating subunits which give nearly linear polymer backbone with the metal center in direct electronic communication with the conjugated polymer backbone. The electrochem. polymn. proceeds through oxidative coupling of pendant thiophenes. The cyclic voltammogram of the Ni complex polymer displays prominent features in the reductive region at -1.35 and -1.75 V assignable to the Ni redox and ligand-centered processes, resp. The polymer shows both metal-centered electroactivity and high cond. and the Ni redox wave does not contribute to the cond. [on SciFinder(R)]
Rotaxane recognition and assembly methods were used to produce new architectures based on a metallorotaxane (Cu, Zn) monomer bearing two independently electropolymerizable groups in the threading unit and the macrocycle host. The controlled assembly and polymn. of the metallorotaxane monomers leads to three-stranded ladder polymers, wherein one of the conjugated chains is sandwiched between two other chains. In this structure the internal polymer behaves as a partially isolated mol. wire when the outer strands are in an insulating state. Neglecting minor effects arising from conformational issues (there are two enantiomeric conformations) the polymer formed from the threading unit is twice as long as the polymer contg. the macrocycle. When the outer polymer chains are insulating, the electroactivity of Cu or Zn ions can assist in interchain transport. [on SciFinder(R)]
A review with 359 refs. is given on conjugated polymers with synthetic receptors and functional groups, biol. sensors, conjugated polymers with entrapped materials to aid in specificity, and unmodified conjugated polymers as sensors. [on SciFinder(R)]
Highly conducting polythiophene-cobalt salen hybrid material catalyzed the redn. of O2. Rotating ring-disk measurements suggested a selective four-electron redn. process took place to produce H2O as the sole product. [on SciFinder(R)]
Molecular wires have progressed from an intellectual curiosity to become the basis for chemical sensors with unprecedented sensitivity. Particularly exciting opportunities are those that make use of biological superstructures to effect conduction through assemblies of molecular wires.[on SciFinder (R)]
A new methodol. is described for the synthesis of poly(p-phenylenebutadiynylene)s based on the Pd/Cu-catalyzed, benzoquinone-mediated homocoupling of terminal acetylenes. Homopolymers synthesized from the 2,5-dialkoxy-1,4-diethynylbenzene monomers 1,4-bis(decyloxy)-2,5-diethynylbenzene and 1,4-bis(hexadecyloxy)-2,5-diethynylbenzene were largely insol., with the sol. portion from the polymn. of 1,4-bis(hexadecyloxy)-2,5-diethynylbenzene exhibiting a no.-av. mol. wt. of 14,000. Completely sol. polymers were obtained from these precursors by the random copolymn. of these monomers. The materials exhibited no.-av. mol. wts. ranging from 67,000 to over 150,000. The UV-visible and emission spectra of these polymers were examd. and found to be very similar to those of structurally analogous poly(p-phenyleneethynylene)s and smaller poly(p-phenylenebutadiynylene)s reported by Kijima et al. [on SciFinder(R)]
A chemosensor design is presented which substantially amplifies the output of a pH-sensitive fluorophore using energy harvested from a conjugated polymer. The construction of thin films was accomplished via layer-by-layer deposition of a new water-sol., cationic poly(p-phenylene ethynylene) (PPE) and an anionic polyacrylate. [on SciFinder(R)]
Cyclization of 1,4-Bis[2-(2-(2-(2-toluene-p-sulfonylethoxy) ethoxy) ethoxy) ethoxy]benzene with 1,4-diiodo-2,5-dihydroxybenzene, followed by coupling with (3,4-ethylenedioxy)-thiophene produced 1,4-Bis((3,4-ethylenedioxy)thiophene)-7,10,13,16,19,26,29,32,35,38-decaoxa[13.13]paracyclophane (I) a highly fluorescent electropolymerizable monomer and electron donor. Addn. of Paraquat to I in CH3CN resulted in formation of a deep-green colored soln. with a charge-transfer absorption band at $łambda$ = 589 nm ($ε$ = 204 M-1cm-1), indicative of the highly electron-donating nature of the thiophene-phenylene-thiophene arom. scaffold. The tetrakis-hexafluorophosphate I-[2]-catenane complex (II) was prepd. by reaction of I with NH4PF6 and 1,4-bis(bromomethyl)benzene. The deep-green complex II exhibits a charge-transfer absorption at $łambda$ = 626 nm ($ε$ = 1230 M-1 cm-1), which is red-shifted relative to that of Paraquat:I complex indicating greater intimacy between donor and acceptor in the [2]-catenane. The crystal structure of II indicates an interlocked $π$-stacked geometry with inner bipyridinium moieties within the cyclophane cavity and outer bipyridinium on the periphery of the cavity. Electrochem. polymn. of I and II proceeds via two propagating sites at the 5-position of 3,4-ethylenedioxythiophene and affords conducting polymers. Oxidn. and redn. potentials for poly-II are identical to those of II monomer suggesting that the neutral polymer backbone has the same electronic influence as the thiophene-phenylene-thiophene in II. For both poly-I and poly-II, the multiple redn. peaks obsd. are indicative of the energetic inequality between the inner- and outer-bipyridinium groups. The cond. of poly-II rapidly reaches a max. of 0.2 S/cm at 0.12 V vs. Fc/Fc+, which decays quickly thereafter. In contrast, the cond. profile for poly-I shows that oxidn. of the backbone occurs over a broad range of potentials without decay. The absorption spectra of both conducting polymers are similar; in the neutral (insulating) form, the $łambda$max for poly-I was 527 nm (2.35 eV) and poly-II 542 nm (2.29 eV). When oxidatively doped, both displayed a longer wavelength band at 767-796 nm (1.62-1.56 eV), indicative of new states formed within the band gap upon reaching a conductive state. Further lower energy absorptions occur at higher oxidn. potentials leading to an absorption at >1100 nm (>1.13 eV) owing to the formation of free carriers. The films differ in that poly-II requires higher oxidn. potentials to reach its conductive state than does poly-I. [on SciFinder(R)]
A cyclophane-based poly(p-phenyleneethynylene) was prepd. and showed to undergo unusual solid-state aggregation to give highly emissive materials. This aggregation was demonstrated to be controllable via the method of film prepn. (Langmuir vs. spin-casting) with facile de-aggregation possible by the introduction of specific analytes or annealing. The subsequent decrease in the fluorescence intensities of spin-cast films after chem.-thermal modifications (due to chain alignment) has potential applications in sensor technologies. [on SciFinder(R)]
The relation between cofacial interpolymer distance and solid-state photophysics of random hydrophilic (Me, iso-Pr, isopentyl)- and hexadecyloxy hydrophobic-substituted poly(p-phenylene ethynylene)s was studied. The side chain bulk influences the packing of the polymers at the air-water interface, by providing greater polymer-polymer spacing. Interchain distance has a strong influence on the spectral properties of PPEs. Thin films were prepd. on glass substrates by drop casting, by spin casting, and by using the Langmuir-Schaefer (LS) method. Normalized UV-vis and PL spectra of the isopropyl-substituted PPV films spin cast, drop, and soln. are similar, illustrating the lack of order in the spin cast film relative to the LS film. However, the drop cast film has a PL max. at 463 nm arising from non-aggregated polymer chains, along with three other red-shifted peaks at 494, 512, and 553 nm which may arise from multiple aggregated states, indicating that the drop cast film is intermediate between the LS (fully aggregated case) and the spin cast (least aggregated state). [on SciFinder(R)]
A novel bent-rod hexacatenar liq. crystal is reported that displays a hexagonal columnar (Colh) phase. The organization of conjugated hexacatenar mesogens in the columnar phase is of interest for their anisotropic electronic properties. The emissive nature of the mesogens varies over the temp. range of the Colh phase and the spectral shifts were analyzed in terms of an exciton-coupling model. The variation of the emission band in this phase is consistent with varying degrees of rotational disorder between the mesogens. The bent-rod shape and highly dipolar nature of the liq. crystal core (mesogen) promotes (as suggested by computation, x-ray diffraction, and photophys. studies) a high degree of antiparallel intermol. correlations between nearest neighbors. The antiparallel organization is novel and differs from structures previously identified in other polycatenars. These studies illustrate the utility of the exciton-coupling model to probe the nature and degree of intermol. correlations in highly dipolar liq. crystals. [on SciFinder(R)]
Two liq. cryst. vanadyl complexes were studied by frequency domain dielec. spectroscopy over the range 10 mHz to 13 MHz. The materials exhibit two or three columnar phases denoted Colro, Colrd, and Colhd that were identified by x-ray diffraction. In the higher temp. Colrd phase, a relaxation process in the kHz range is obsd. that is attributed to the reorientation about the mol. short axis. A pronounced dielec. relaxation process shows up in the low temp. Colro phase at hertz and sub-hertz frequencies. This slow relaxation is assigned to reorientation of the mol. dipoles within the polar linear chains, which are aligned along the column's axis. Triangular wave switching studies at low frequency reveal processes inside the Colro phase which are most probably due to ionic/charges relaxations but a ferroelec. switching for an achiral discotic system cannot be ruled out completely. Below the Colro phase there is an orientationally disordered cryst. Crx phase with disordered side chain dipoles. A dielec. relaxation process connected with the intramol. relaxation of the alkoxy side chains, similar to the $\beta$-process of polymers, was found in the lower temp. Crx phase. [on SciFinder(R)]
The syntheses of two novel iptycene monomers, 1,4-diiodotriptycene (I) and 2,3-diiodo-4,9-dihydro-4,9-benzonaphtho[2,3,c]thiophene (II), are described herein. These monomers were subsequently copolymd. with a no. of diethynylphenyl monomers via a Sonogashira-Hagihara coupling to afford both regiodefined polymers and random terpolymers. Terpolymers derived from coupling I and 2,5-dihexadecyloxy1,1,4-diiodobenzene with a diethynylpentiptycene exhibit emission spectra that are only slightly perturbed from soln. to the solid state, suggesting that polymer assocn. is effectively inhibited in condensed phases. An äll-iptycene\" polymer derived from the copolymn. of II with a diethynylpentiptycene monomer is also notable in that it owes its soly. to a combination of its nonlinearity and the presence of rigid iptycene groups rather than to flexible side chains. [on SciFinder(R)]
Doping of the ferroelec. Sm-C* phase with bent-shaped mols. induces the antiferroelec. Sm-C*A phase. The effect was obsd. by electrooptic and dielec. measurements in systems with weak interlayer interactions in which the relative strength of anticlinic-synclinic order between mols. in adjacent layers is easily controlled by external factors. FTIR spectroscopy studies suggest that the bent-shaped mols. are not flat. They reorient upon the elec. field-induced antiferroelec.-ferroelec. transition to adopt a position in which the av. direction of the carbonyl groups is in the smectic plane and a bending tip along the C2 symmetry axis. [on SciFinder(R)]
Prepn. and photophys. characterization of three polydiacetylene derivs. are discussed with the emphasis on the role of interchain spacing within two-dimensional Langmuir monolayer assemblies. Photophys. and geometrical properties and normalized UV visible and photoluminescence of these films are also discussed. [on SciFinder(R)]
The prepn. and cond. measurements of the title polymer were discussed. The cond. studies showed a large hysteresis suggesting that a mol. level compression took place. Thus the materials based on this polymer should have potential use in actuating devices or as artificial muscle. [on SciFinder(R)]
1997
The authors present novel light-emitting devices based on several pyridine-contg. conjugated polymers and copolymers in various device configurations. The high electron affinity of pyridine-based polymers improves stability and electron transport properties of the polymers and enables the use of relatively stable metals such as Al as electron injecting contacts. Bilayer devices utilizing poly(9-vinyl carbazole) (PVK) as a hole-transporting/electron-blocking polymer show dramatically improved efficiency and brightness as compared to single layer devices. This is attributed to charge confinement and exciplex emission at the PVK/emitting polymer interface. The incorporation of conducting polyaniline network electrode into PVK reduces the device turn-on voltage significantly while maintaining the high efficiency. Two novel device configurations that enable the use of high work function metals as electrodes are pointed out. [on SciFinder(R)]
The photoinduced electron transfer between conjugated polymers and a series of functionalized fullerenes was studied. A new photoluminescence signal was obsd. in the near IR (∼1.4 eV). This weak IR photoluminescence does not result from direct excitation of the fullerene, but from radiative electron-hole recombination between the fullerene excited state and the polymer ground state. The intensity of this recombination luminescence depends on the electrochem. nature of the functional group; it is obsd. only for fullerenes with first redn. potential higher than that of C60. [on SciFinder(R)]
In this paper polymers are described of two metallorotaxane systems, Rot(1,M) and Rot(2,M) (M = Zn2+ or Cu1+), which are formed by complexing a macrocyclic phenanthroline, a 5,5'-bis([2,2'-bithiophen]-5-yl)-2,2'-bipyridine (ligand 1) or 5,5'-bis(3,4:3',4'-bis(ethylenedioxy)[2,2'-bithiophen]-5-yl)-2,2'-bipyridine (ligand 2), and Zn2+ or Cu1+ ions. The corresponding polymetallorotaxanes, PolyRot(1,M) and PolyRot(2,M), are produced by oxidative polymn. of Rot(1,M) and Rot(2,M). Investigations of the electrochem., conducting, and optical properties of the metallorotaxanes and polymetallorotaxanes as well as related nonrotaxane polymers Poly(1) and Poly(2) are reported. The combined electrochem. and cond. studies of PolyRot(1,M) and PolyRot(2,M) indicated that the polymetallorotaxane's redox and conducting properties were dramatically affected by the Lewis acidic and redox properties of the coordinated metal ions. The Lewis acidity produces charge localization and a redox conduction process in both polymetallorotaxane systems. The matching of the polymer and Cu1+/2+ couple redox potentials in PolyRot(2,Cu) resulted in a Cu1+/2+ contribution to cond. The metal-free PolyRot(1) and PolyRot(2) were produced by extg. the metal ions, and these polymers reversibly bound Zn2+ or Cu2+ ions in soln. The Cu2+ dopes the films of PolyRot(2) and Poly(2), which have lower oxidn. potential than those of PolyRot(1), to produce 106-107-fold cond. increases. In the case of PolyRot(1) and Poly(1), the rotaxane structure was demonstrated to be key for reversible complexation of metal ions. [on SciFinder(R)]
A versatile method for the synthesis of complex, fused, polycyclic, arom. systems in high chem. yield is described. Construction is achieved using a general two-step synthetic sequence. Pd-catalyzed Suzuki and Negishi type cross-coupling chemistries allow for the prepn. of non-fused skeletal ring systems in yields consistently >80%. The crit. ring-forming step, which generally proceeds in very high to quant. yield, utilizes (4-alkoxyphenyl)ethynyl groups and is induced by strong electrophiles such as trifluoroacetic acid and iodonium tetrafluoroborate. The reaction in essence produces phenanthrene moieties which are integrated into extended polycyclic arom. structures. Fused polycyclic benzenoids as well as benzenoid/thiophene systems may be prepd. by this methodol. The scope of the described cross-coupling/cyclization chem. including mechanistic insights and problematic side reactions are described. [on SciFinder(R)]
The authors study the photophys. properties of the pyridine-based polymers poly(p-pyridyl vinylene) (PPyV) and poly(p-pyridine) (PPy). The primary photoexcitations in the pyridine-based polymers are singlet excitons. The authors observe direct intersystem crossing (ISC) on picosecond time scales with the vol. d. of triplet excitons varying with the sample morphol. (film or powder). These effects are demonstrated clearly by examg. the millisecond photoinduced absorption characteristics of powder and film forms of PPyV. The pyridine-based polymers were shown to be promising candidates for polymer light-emitting devices, both conventional diode device and sym. configured ac light-emitting (SCALE) device. Here the authors examine the role of insulating layers and their interfaces with the emitting layer and electrodes in the SCALE device operation, with emphasis on the central role of the polymer-polymer interfaces. [on SciFinder(R)]
A review, with 12 refs., is given on the use of conjugated polymers for the development of new sensory methodologies. [on SciFinder(R)]
Single-mol. fluorescence spectroscopy of a multichromophoric conjugated polymer (mol. wt. ∼ 20,000) revealed surprising single-step photobleaching kinetics and acute jumps in fluorescence intensity. These jumps were shown not to result from spectral diffusion and were attributed to fluctuations in the quantum yield of emission for the mols. The data indicate efficient intramol. electronic energy transfer along the polymer chain to a localized fluorescence-quenching polymer defect. The defects are created by reversible photochem. of the polymer. These findings have implications for the use of conjugated polymers in light-emitting diode displays and sensors. [on SciFinder(R)]
Photoluminescence and electroluminescence spectra were obtained, of heterojunctions formed from poly(vinyl carbazole) (PVK) and poly(pyridyl vinylene phenylene vinylene) (PPyVPV) conjugated polymers. Bilayers of PVK and PPyVPV show a photoluminescence peak which cannot be assigned to either the PVK or the PPyVPV layer. Absorption spectra show that the addnl. feature results from an exciplex at the bilayer interface. The electroluminescence spectrum from the heterojunctions is due to exciplex emission, with internal efficiency of ∼0.1-0.5%. [on SciFinder(R)]
The emitting properties of polypyridine and various copolymers were measured and prototype light-emitting devices based on pyridine-contg. conjugated polymers and copolymers were assembled in various configurations. The high electron affinity of pyridine based polymers enables the use of relatively stable metals such as Al or even ITO as electron injecting contacts. Bilayer devices utilizing poly(9-vinyl carbazole) (PVK) as hole transporting/electron blocking polymer were assembled, which show improved efficiency and brightness, due to charge confinement and exciplex emission at the PVK/emitting polymer interface. The incorporation of a conducting polyaniline network electrode in the PVK structure, lowers the device turn-on voltage significantly while maintaining high efficiency. Control of aggregate formation in the polymer films by blending with insulating host polymers [PMMA] opens up the possibility of making voltage-controlled multi-color light-emitting devices. [on SciFinder(R)]
The low energy photophysics of the pyridine-based polymers poly(p-pyridine)(PPy), poly(p-pyridylvinylene) (PPyV) and copolymers made up of PPyV and poly(p-phenylenevinylene) (PPyVPV). The absorption and luminescence properties are morphol. dependent. The primary photoexcitations within these polymers are singlet excitons which may emit from individual chains following a random walk to lower energy segments, depending upon the excitation energy. Films display red shifted absorption and emission properties with a decrease in photoluminescence efficiency which can be attributed to aggregate formation in comparison to powder and soln. forms. Photoinduced absorption (PA) studies show direct conversion of singlet to triplet excitons on the ps time scale. Polaron signatures and the transition between triplet exciton states are seen in powder forms using ms PA techniques. Film forms display only a polaron signature at millisecond times indicating that morphol. plays a key role in the long-time photophysics for these systems. Photoluminescence detected magnetic resonance studies also have signatures due to both polarons and triplet excitons. The size of the triplet exciton is limited to a single ring suggesting that the triplet exciton may be trapped by extrinsic effects. [on SciFinder(R)]
Poly(pyridylvinylenephenylenevinylenes) were synthesized by Heck coupling procedures. These materials display large red shifts in their optical absorption which upon protonation of alkylation of the pyridyl nitrogen. Some of the polymers were found to be liq. cryst. The protonated or alkylated versions exhibit highly organized structures due to charge-transfer interactions between polymer chains. [on SciFinder(R)]
The fabrication is reported of color variable bipolar/a.c. LEDs based on conjugated polymers. The devices consist of blends of pyridine-phenylene and thiophene-phenylene based copolymers sandwiched between the emeraldine base form and the sulfonated form of polyaniline. ITO and Al are used as electrodes. The devices operate under either polarity of driving voltage with different colors of light being emitted, red under forward bias, and green under reverse bias. The relative fast time response allows the rapid switching of colors and a.c. operation. [on SciFinder(R)]
Photoluminescent and electroluminescent studies of bilayer heterojunctions formed from poly(pyridyl-vinylene-phenylene-vinylene) (PPyVPV) derivs. and poly(vinyl carbazole) (PVK) show an emission peak which cannot be ascribed to either the PPyVPV or PVK layer. This peak results from an exciplex at the bilayer interface as demonstrated through studies of absorption and photoluminescence excitation spectra. The photoluminescence efficiency of the exciplex is greater than 20%. The electroluminescence spectrum from the bilayer devices is entirely due to exciplex emission, with internal efficiencies initially achieved exceeding 0.1%. [on SciFinder(R)]
The syntheses and mesomorphism of novel octaalkoxymethyl-substituted tetra-2,3-thiophenoporphyrazines, heterocyclic phthalocyanine analogs in which the benzene rings are replaced by thiophene rings, are reported. NMR anal. indicates that these materials exist as isomeric mixts. due to the asymmetry induced by the thiophene ring. Among the attractive features of liq.-cryst. 2,3-thiophenoporphyrazines over phthalocyanines are their lower mesomorphic and isotropic temps. These features resulted in liq. crystallinity at room temp. Similar to liq.-cryst. phthalocyanine derivs., metalation with Cu raises the transition temps. [on SciFinder(R)]
1998
A review, with 24 refs., is given on conceptual aspects of how mol. wires (conjugated polymers) can be used to amplify mol. chemosensors. The properties that are responsible are universal and can be utilized in a multitude of schemes. The sensitivity and diversity available suggest that these materials will be important for future sensor technologies. [on SciFinder(R)]
The synthesis and the electrochem. properties of 2 polymer-transition metal hybrids are reported. The oxidn. potential of the Co-contg. N,N-ethylenebis(salicylidenimine) polymer films was investigated by cyclic voltammetry, and the influence of the film thickness on the redox potential was analyzed. The test results for both polymers are shown and discussed in detail. It was concluded from the exptl. data, that the Co-polymer hybrids display a high cond. and sensitivity to coordinating ligands. Moreover, possible application fields for the hybrid materials are outlined. [on SciFinder(R)]
Mixts. of Mo2(O2C(CH2)nCH3)4, where n = 6 (=Oct) and 7 (=Non), form a columnar mesophase upon heating and the crystal-to-liq. crystal phase transition temps. differ little from those predicted by the Shroder-van Laar equation for an ideal mixt. The XRD of the solid sample obtained from cooling a 1:1 mixt. of Mo2(Oct)4 and Mo2(Non)4 conformed to a triclinic cell with lattice parameters intermediate between those of the pure compds. Similarly in the mesophase the intercolumnar sepn. (d) was an intermediate distance. The phase behavior of mixts. of Mo2(non)4 and Mo2(O2C(CH2)10CH3)4 [Mo2(Dod)4] were more complex, but in all instances a mesophase was obsd. whereas that of the pure Mo2(Dod)4 shows only a cryst. solid to isotropic phase transition. Mixts. of Mo2(Oct)4 and Mo2(O2C(CF2)6CF3)4 [Mo2(Octf)4] also form a columnar mesophase with an intercolumnar sepn. intermediate between that of the pure compds. The mesophase is an optically pos. material with the largest component of the index of refraction coincident with the columnar axis. The solid to mesophase transition temps. varied significantly from that predicted for an ideal mixt. These results are discussed from facile carboxylate scrambling to produce Mo2(O2CR)4-n(O2CR')n, where n = 0-4 in both the mesophase and the resultant solid soln. [on SciFinder(R)]
Nondiscoid Rh and Ir dicarbonyl $\beta$-diketonate complexes organize in columnar liq. crystal phases with hexagonal disordered structures (Colhd). The shape and dipolar attributes of these materials produce highly correlated antiparallel pairwise arrangements. This organization was studied by x-ray diffraction and miscibility studies. The liq. crystallinity is stabilized by decreasing the no. of C atoms in the side chains, and single component room-temp. liq. crystals were developed. The nature of the metal is also important, and Ir mesogens exhibited higher clearing points than the analogous Rh materials. [on SciFinder(R)]
Optical absorption and photoluminescence spectroscopy have been carried out on a new class of (phenylene) ring substituted p-pyridylvinylene phenylenevinylene polymers used as active materials in light emitting diodes. The effects of the ring substitutions on the optical absorption and photoluminescence energies are qual. explained through the use of semiempirical quantum chem. modeling of the ring torsion angles. Reduced aggregation through the use of \"strap\" substituents on the phenylene rings also is discussed. [on SciFinder(R)]
We present photoluminescence and electroluminescence studies of bilayers and blends formed from poly(vinyl carbazole) (PVK) and poly(pyridyl vinylene phenylene vinylene) (PPyVPV) copolymer derivs. Bilayers of PVK and the PPyVPV copolymers have a photoluminescence emission which cannot be assigned to either the photoluminescence of PVK or the PPyVPV layer. The blends of the two polymers show a similar new photoluminescence emission for a large range of concns. Absorption and photoluminescence excitation spectra confirm that the addnl. feature is an excited state species which results from an exciplex at the polymer/polymer interface. Bilayer light-emitting devices utilizing the PPyVPV copolymers show an electroluminescence spectrum consistent with emission from the exciplex. The efficiency of the bilayer devices as compared to single layer devices increases by over three orders of magnitude due to the exciplex formation and the elimination of exciton formation near the luminescence quenching electrodes. [on SciFinder(R)]
An efficient one-pot procedure has been developed for the prepn. of rigid non-fluxional dialkoxy tungsten calix[4]arenes I [HOCHRCHROH = ethyleneglycol, methylcatechol, (S)-(-)-1,1'-bi-2-naphtho
An organometallic coupling electrophile-induced cyclization strategy for the synthesis of p-terphenyl compds. has been extended to the synthesis of p-quinquephenyl systems. In this work the authors report the synthesis of various polycyclic arom. systems contg. nine annelated rings including the synthesis of functionalized polycyclic arom. systems. An interesting side reaction which leads to an indenyl spiro ring system is also described. This side reaction can be suppressed by changing the electrophile (from H+ to I+) or by modification of the cyclization precursor. The UV-vis and fluorescence spectra of several of these polycyclic aroms. and the p-quinquephenyl precursors are also reported. [on SciFinder(R)]
The synthesis, spectroscopy, and fluorescence quenching behavior of pentiptycene-derived phenyleneethynylene polymers, 1-3, are reported. The incorporation of rigid three-dimensional pentiptycene moieties into conjugated polymer backbones offers several design advantages for solid-state (thin film) fluorescent sensory materials. First, they prevent $π$-stacking of the polymer backbones and thereby maintain high fluorescence quantum yields and spectroscopic stability in thin films. Second, reduced interpolymer interactions dramatically enhance the soly. of polymers 1-3 relative to other poly(phenyleneethynylenes). Third, the cavities generated between adjacent polymers are sufficiently large to allow diffusion of small org. mols. into the films. These advantages are apparent from comparisons of the spectroscopic and fluorescence quenching behavior of 1-3 to a related planar electron-rich polymer 4. The fluorescence attenuation (quenching) of polymer films upon exposure to analytes depends on several factors, including the exergonicity of electron transfer from excited polymer to analytes, the binding strength (polymer-analyte interactions), the vapor pressure of the analyte, and the rates of diffusion of the analytes in the polymer films. Films of 1-3 are particularly selective toward nitro-arom. compds. The dependence of fluorescence quenching on film thickness provides an addnl. criterion for the differentiation of nitro-arom. compds. from other species, such as quinones. In short, thinner films show a larger response to nitro-arom. compds., but show a lower response to quinones. Such differences are explained in terms of polymer-analyte interactions, which appear to be electrostatic in nature. The rapid fluorescence response (quenching) of the spin-cast films of 1-3 to nitro-contg. compds. qualifies these materials as promising TNT chemosensory materials. [on SciFinder(R)]
The emission and absorption characteristics of a conjugated poly(phenylene bithiophene) and a monomeric model compd. were investigated as a function of [Li+], [Na+], [K+], and [Ca2+]. The calix[4]arene bithiophene receptor that is present in both compds. provides selectively for Na+ and the absorption and emission characteristics are not affected by Li+, K+, or Ca2+. Both systems display absorption spectra which are relatively insensitive to Na+; however, the Stokes shift of the emission is reduced by added Na+•. For the model system, increasing [Na+] provides a shift of the emission that is consistent with an equil. mixt. of bound and unbound receptor. The polymer displays a larger shift in the emission in response to Na+ and due to multiple binding sites lacks an isoemissive point. The chain length of the polymer also has an effect on this behavior. This behavior may be due to energy migration to regions of the polymer which do not have bound Na+ and can relax to lower energy conformations. This description is also borne out by the redn. in the lifetimes of the excited states with increasing [Na+] for both the polymer and the model system. This mechanism may provide a route to systems which can function as digital indicators at crit. concns. of analytes. [on SciFinder(R)]
Spin-cast films of a pentiptycene-derived phenyleneethynylene polymer, 2, display a fast fluorescence response (seconds) to vapors of 2,4,6-trinitrotoluene (TNT), 2,4-dinitrotoluene (DNT), and 1,4-benzoquinone (BQ). The fluorescence attenuation of 2 is dependent on the time of exposure to these quenchers and on the thickness of thin films. Thinner films show a better response to TNT and DNT but the opposite is true for BQ. Such differences are attributed to different polymer-analyte interactions. For comparison, corresponding studies on an alkoxy-substituted phenylenethynylene polymer, 3, were also carried out. The results indicate that 2 is superior to 3 as a fluorescent chemosensor in terms of sensitivity, selectivity, solvent soly. and solvent stability. [on SciFinder(R)]
1995
Soln.-cast films of four different poly(2,5-dialkoxy-p-phenyleneethynylene) mols. with varying backbone chain lengths and varying alkoxy substituent chain lengths and 1,4-diethynyl-2,5-dihexadecyloxybenzene-9,10-dibromoanthracene alternating copolymer (I) have been characterized electrochem. and by x-ray diffraction and DSC. The polymers have varying degrees of order and crystallinity based on long-range lamellar structure. Cyclic voltammetry in liq. SO2/electrolyte shows that the onset of oxidn. for poly(2,5-dialkoxy-p-phenyleneethynylene) occurs at ∼1.05 V vs SCE with the more cryst. polymers having slower electrochem. response than the less cryst. ones. In situ characterization of the potential dependence of cond. in the same medium shows that the max. conductivities of poly(2,5-dialkoxy-p-phenyleneethynylene) range from ∼0.2 to ∼5 $Ømega$-1 cm-1, suggesting that higher cond. is assocd. with lower long-range order in the polymer films but showing little dependence on av. polymer chain length. Poly(2,5-dialkoxy-p-phenyleneethynylene) all have max. cond. at ∼1.6 V vs SCE and finite potential windows of high cond. ∼0.55 V wide, indicating that the potential of max. cond. and the width of the window of high cond. are detd. by mol. rather than bulk properties. For I, the onset of oxidn. occurs at ∼0.8 V vs SCE, the potential of max. cond. is ∼1.5 V vs SCE, and the width of the potential window of high cond. is ∼0.85 V. [on SciFinder(R)]
We have fabricated unilayer electroluminescent devices from sol. poly(p-pyridine) (PPy). The soly. of PPy in weak acids allows direct spin casting of the polymer films. The electroluminescence spectrum peaks at 2.5 eV (497 nm) corresponding to white light weighted towards the blue end of the spectrum. The photoluminescence spectrum peaks at 2.35 eV (530 nm). The operating voltages of the devices ranged from 4 to 12 V with current densities of 6 to 8 mA/mm2. We compare our devices with similar blue emitting devices based on poly(p-phenylene). [on SciFinder(R)]
This paper presents the synthesis of N,N,N',N'-tetraethyl-1,4-phenylenediamines bridged by ethynyl-phenyl-ethynyl and diethynyl linkages. These compds. are of interest to det. if the N,N,N',N'-tetraethyl-1,4-phenylenediamines exhibit delocalized mixed oxidn. states when oxidized electrochem. 1-Butoxy-2,5-bis(N,N-diethylamino)-4-ethynylbenzene was a key intermediate, and nucleophilic addn. of lithium (trimethylsilyl)acetylide to 2,5-bis(N,N-diethylamino)-1,4-benzoquinone provided an efficient synthesis of this compd. This intermediate was then assembled into the redox assemblies of interest in high yield by use of palladium-catalyzed cross-coupling and copper-catalyzed oxidative homo-coupling protocols. Cyclic voltammetry of these compds. indicate that the N,N,N',N'-tetraethyl-1,4-phenylenediamine redox units behave independently and that the only interactions are electrostatic. As a result, we conclude that the electrochem. generated radical cations and dications are highly localized. [on SciFinder(R)]
The poly(p-phenyleneethynylene) mols. synthesized by a Pd-catalyzed cross-coupling reaction of diiodobenzene derivs. and derivs. of 1,4-diethynylbenzene, are highly luminescent materials. The polymers are sol. by virtue of the -OC16H33 groups introduced on the arom. rings and by controlling their mol. wt. The fluorescent quantum yields are between 0.35 and 0.40 depending on the material. The excited-state lifetimes of the polymers are 1-2 ns, slightly shorter than that of the model compd. 1,4-diphenylethynyl-2,5-dibutoxybenzene, which has a lifetime of 3 ns. Incorporation of anthracene, coupled at the 9,10-positions by using 9,10-dibromoanthracene, into the polymer backbone decreases the quantum yield to between 0.05 and 0.27 depending on the anthracene content. In addn., low-energy electronic transitions and lower wavelength emission bands assocd. with the anthracene group are produced. The polymers harvest optical energy and transfer it to the anthracene resulting in emission from this chromophore. In the case where only terminal anthracene units, introduced by using 9-bromo-10-phenylethynylanthracene, are present, the process is very efficient with >95% of the energy being transferred to the end groups. [on SciFinder(R)]
The authors demonstrate herein how conjugated polymers (mol. wires) can be used to interconnect (wire in series) receptors to produce fluorescent chemosensory systems with sensitivity enhancements over single receptor analogs. The enhancement mechanism in the polyreceptor materials is based on an energy migration scheme in which excitations, diffuse along the polymer backbone. Analyte binding produces trapping sites for the excitations which results in greatly attenuated emission intensity. Three different cyclophane-based receptor systems that bind paraquat were studied. These systems are quenched by paraquat binding, and the quenching enhancements relative to a monomeric model compd. were used to det. the efficiency of energy migration. Two polymers with related poly(phenyleneethynylene) structures were studied, and the all-para system was found to exhibit more facile energy migration than the more electronically localized analog that contained meta linkages. The para polyreceptor system was found to display a 65-fold enhancement in sensitivity to paraquat as compared to a model monoreceptor fluorescent chemosensor. However, delocalization alone is not sufficient to produce facile energy migration, and the more delocalized polythiophenes appear to be less effective at energy migration than the para poly(phenyleneethynylene) material. Paraquat-induced fluorescent quenching studies on homologous polymers that lacked the cyclophane receptors were also performed. Diffusive quenching by paraquat is enhanced by energy migration. [on SciFinder(R)]
The authors report the synthesis, optical, and electrochem. properties of a calix[4]arene-substituted polythiophene which demonstrates ion-selective voltammetric, chromic, fluorescent, and resistive responses. The ionochromic response of this polythiophene on exposure to Na+ shows an increased effective conjugation length of the polymer backbone. Despite this, Na+ induces a large pos. shift in the potential at which the polymer is oxidized (greater than +100 mV) commensurate with a large decrease in cond. (>99%). Although the calix[4]arene-substituted polythiophene exhibits no changes in the UV-visible spectrum and only minimal changes in the voltammetric responses on exposure to Li+ or K+, there are large decreases in relative conductivities (69 and 47%, resp.). Thus, although the sensory properties of this polymer are expressed via several measurable entities, the ionoresistive response is clearly the most sensitive. This sensitivity originates from the cooperative nature of carrier transport in a conducting polymers (CP) and is thus inherent in chemoresistive CPs. [on SciFinder(R)]
The authors report conducting polymer-based sensors which transduce reversible, noncovalent, and non-redox-dependent mol. recognition events into measurable changes in cond. These chemoresistive polymers are derived from bithiophenes contg. cyclophane receptors capable of forming self-assembled pseudorotaxane complexes with paraquat. The electrostatic perturbations arising from pseudopolyrotaxane formation cause a decrease in carrier mobility and thus lower the cond. The chemoresistive response was consistent with decreased carrier mobility and exhibited an enhanced sensitivity to analyte-promoted electrostatic perturbations relative to the voltammetric response. Polymer-based devices which demonstrate a real time chemoresistive response to paraquat are also reported. [on SciFinder(R)]
A new method for the enhancement of a fluorescent chemosensory response using conjugated polymers is reported. This method makes use of the delocalized electronic structure of these materials which allows the excited state to migrate to an occupied receptor site. In the conjugated polymer I each repeating unit displays a cyclophane receptor which binds paraquat (Ka ≈ 1600 M-1). The binding of paraquat to I and II results in electron transfer quenching. The relative amt. of quenching in I is enhanced relative to II due to energy migration to the occupied sites. Theor., I can exhibit a max. sensitivity which is n times greater than II where n is the d.p. This effect leads to an enhancement in the static quenching consts. as detd. by the Stern-Volmer relation from 1600 M-1 for I to 105,000 M-1 for II (Mn = 122,500). Polymers lacking receptor groups also displayed enhanced dynamic quenching consts. relative to monomeric analogs. [on SciFinder(R)]
The azo-substituted calix[4]arene I (R = N:N-C6H3-(O(CH2)10H)2-3,4) in pure form does not display a stable thermotropic mesophase. A novel method is reported for stabilization of mesomorphic behavior by making use of the calix[4]arene's receptor properties. In particular host-guest interactions between I and 1,4-(diformamide)butane (II) stabilize a bowlic hexagonal columnar phase (Bh) with very low clearing enthalpies and high fluidity. X-ray diffraction studies indicate that the Bh phase is the only mesophase present at I:II ratios of 2:1, but at ratios of 4:1 and 1:1 this phase coexists with small amts. of other unidentified phases. This system also displays the unusual properties that the range of mesomorphism is independent of the host/guest ratio and that only a fractional amt. of guest is required to stabilize the mesophase. The latter feature is indicative of a cooperative transition to the mesomorphic state. [on SciFinder(R)]
Octahedral liq. crystals based upon diketonate complexes of Fe+3, Mn+3, and Cr+3 are reported. These materials display columnar phases with ≥12 side chains and an ordered smectic phase with 6 side chains. [on SciFinder(R)]
The synthesis of conjugated polymers incorporating recognition elements for ions and electron deficient org. mols., e.g., polythiophenes, polyphenylenes, etc., are described. Palladium [Pd(PPh3)4] catalyzed coupling of polythiophenes which form a self-assembled pseudopolyrotaxane and synthesis of conjugated polymers with enhanced chemosensory response are described. The use of these methods for the construction of all carbon ladder polymers, i.e., graphite ribbons is outlined. [on SciFinder(R)]
The electroluminescence properties of three poly(pyridylvinylene) regioisomers used for LED applications are studied. [on SciFinder(R)]
Ladder polymers are prepd. by postpolymn. cyclization of acetylene group-contg. polyarylenes. [on SciFinder(R)]
For polypyridine the Stokes shifted fluorescence spectrum and photoinduced absorption studies suggest that triplet excitons are importance for this system. We have fabricated electroluminescent devices with polypyridine as the emitting layer. The electroluminescence and photoluminescence spectra are similar and are peaked in the blue-green region. The simplicity of synthesis, the ability to cast polypyridine directly from soln., and the ability to quaternize the nitrogen site makes polypyridine of particular interest. [on SciFinder(R)]
The prepn. of head-to-head (I), head-to-tail (II), and random (III) regioisomeric forms of the title compd. is described. Electrochem. data from cyclic voltammograms are reported. Methylation to give the quaternary regioisomers resulted in a decrease in cond. for the reduced forms of I and III isomers (to <10-7 S/cm). The quaternary II isomer had a cond. of \~{}2.5 x 10-5 S/cm. [on SciFinder(R)]
A novel class of photo- and electroluminescent polymers (poly(p-pyridyl vinylene), poly(p-pyridinium vinylene), and their butyl-substituted analogs), and their application in org. light emitting devices (OLED) are reported. Polymer thin films for OLEDs were prepd. by both spin coating and a new self-assembly technique on a variety of substrates. They exhibit good fluorescence quantum yields, and the Bu derivs. are sol. in org. solvents. A device consisting of a 50-nm poly(p-phenylenevinylene) layer as hole conductor between the In/Sn oxide-coated glass support and the Bu poly(p-pyridyl vinylene) film and capped with an Al cathode operates with good quantum efficiency (5 × 10-4 photons/electron) for several hours. [on SciFinder(R)]
Oxovanadium-based surfactants which display binary (surfactant/H2O) and ternary (surfactant/H2O/decanol) mesomorphism at room temp. are reported. The phase behavior of the ternary system was studied and both hexagonal and lamellar phases were identified.the investigated ligand systems are based on a tetra-anionic diamidate-diphenolate ligand which forms dianionic oxovanadium complexes with high stability in aq. environments. [on SciFinder(R)]
A systematic study of the liq.-cryst. properties of 30 metal bis($\beta$-diketonate) complexes (M = Cu, VO, Pd) that exhibit discotic mesophases is reported. This study has detd. that the ability of the metal center to influence the mesophase stability depends upon the d. of side chains. In the 10-side-chain complexes I, all of the materials were liq. cryst. In this series the M = VO analogs have lower melting and clearing points than those with M = Cu and M = Pd. For the 12-side-chain series II the opposite is true, and the M = VO materials have substantially higher clearing points. The differences between series I and II arise from the enhanced core-core interactions that accompany the increased side-chain d. The side-chain-induced organization assists the expression of the metal center's character in detg. the stability and nature of the mesophase. The fact that the transition temps. of the M = Cu and M = Pd compds. differ more in series II than in series I is also a manifestation of this greater organization. The influence of the metal centers is discussed in the context of intermol. dative assocns. and for some phases of the M = VO materials these interactions produce polymeric (-V:O-V:O-)n structures. [on SciFinder(R)]
1996
5,5'-Bis(2''-bithiophene)-2,2'-bipyridine ligand (L) and its Ru complex [Ru(L)3(PF6)2] were synthesized and underwent polymn. upon electrochem. oxidn. The resultant polythiophene-Ru(L)3n+ hybrid material displayed both the conducting properties of the polymer and the redox properties of the isolated metal centers which was shown by cyclovoltammetry. [on SciFinder(R)]
The chiral metallocalix[4]arene [W(L)Cl2] (L = 3,4-dimethylcalix[4]arene) was resolved by means of tungsten alkoxide complex prepn. The dichloride was reacted with (1S,2S)-trans-1,2-cyclohexanediol or (S,S)-(-)-hydrobenzoin to give the substituted complexes. The hydrobenzoin complex diastereomers were more easily sepd. than those of the cyclohexanediol complex and were converted back to the dichlorides or to the oxides [WO(L)] as pure enantiomers. The CD spectra of the thus prepd. [W(L)Cl2] complexes are mirror images, confirming that they are enantiomers. [on SciFinder(R)]
Bowl-shaped chromophores fabricated with tungsten and molybdenum oxo complexes of calix[4]arenes substituted at the para positions with phenyldiazenyl (R2C6H3N2) derivs. are investigated. The nature of the lowest energy excited states has been addressed theor. using EHMO calcns. and exptl. using UV-visible, luminescence, and polarized emission spectroscopy at 77 K. The lowest energy excited states are intraligand (azobenzene fragment) $π$$π$* (S2 and T2) and n$π$* states (S1 and T1). The compds. are weakly luminescent in the 600-630 nm range exhibiting emissions arising from the 1($π$$π$*) states (S2 → S0, 1 < $\tau$e < 4 ns, and 3 × 10-3). The region located above 900 nm was not investigated. Evidence for guest-host chem. has been demonstrated by 1H-NMR spectroscopy, and the binding consts. for two of the compds. with pyridine in toluene as a solvent were obtained by UV-visible spectroscopy. The presence of a guest mol. inside the luminescent metal oxo complex of a calix[4]arene hosts decreases the emission lifetimes. [on SciFinder(R)]
Cu(bis-$\beta$-diketonate) complexes are reported which display columnar liq.-crystal phases with a hexagonal disordered structure (Dhd). The complexes do not have the disk-shape characteristic of most Dhd materials, but produce a disk shape by forming dimers with 90° rotations between nearest neighbors. In the liq.-cryst. state this dimerized nature produces short-range rotational correlations. Three side-chain Cu bis-$\beta$-diketonates with a single Ph substituent are not liq. cryst., and an extension of the mesogenic core is necessary to introduce liq. crystallinity. The simple Ph analogs are monotropic, and the addn. of electron-withdrawing substituents to the Ph moiety results in a stabilization of the mesophase. These substituents produce favorable dipolar interactions which stabilize the mesophase. Consistent with this explanation, electron-donating substituents are not effective at stabilizing the mesophase. Substitution of the complex with thiophene groups rather than phenyls produces stable mesophases with greatly lowered melting and clearing points. This latter result indicates that thiophene substitution provides dispersive forces which destabilize the crystal phase. Thiophene substitution may provide a general method for reducing transition temps. in metallomesogens. [on SciFinder(R)]
This paper describes the first synthesis of 3-methylcalix[4]arene, an inherently chiral mol. which can be readily functionalized. The procedure involves hydromethylation of the readily available 4-bromo-3-methylphenol followed by dehydrative cyclization with TiCl4 to give 4-bromo-3-methylcalix[4]arene. Debromination proceeds in near quant. yield to give the targeted 3-methylcalix[4]arene-25,26,27,28-tetrol. 3-Methylcalix[4]arene undergoes reactions at the para position to give functionalized chiral calix[4]arenes in good to excellent yields. [on SciFinder(R)]
Two metallo-rotaxane complexes, assemble when the macrocyclic phenanthroline and Cu+ or Zn2+ ions are combined in CH2Cl2. Anodic electrochem. polymn. produce the corresponding polymetallorotaxanes. Electrochem. investigations indicate that the conductivities of the polymers exhibit redox-like profiles. It was demonstrated that the polyrotaxane structure reversibly bind Zn2+ ions, thereby confirming the potential of the structures as sensory materials. [on SciFinder(R)]
The unusual electronic and optical properties of many electroluminescent and conducting polymers arise from extended conjugation along the polymer backbone, which can also lead to insoly., aggregation, and gelation. Synthetic efforts to produce an optimal structure require a balance between the persistence length and the effective conjugation length for the successful implementation of these materials in photonic and electronic devices. The soln. properties of a group of poly(phenyleneethynylenes) were investigated using a variety of light scattering techniques, including polarized and depolarized intensity measurements, dynamic light scattering, and size exclusion chromatog. with a multiangle light scattering detector (SEC/LS). Interpretation of light scattering in the presence of absorption, fluorescence, and optical anisotropy is discussed. The mol. wts. detd. by light scattering encompassed the range from 10 × 105 to 5 × 106, with the root-mean-square radius of gyration as high as 250 nm. The results may be interpreted with a wormlike chain model to yield a persistence length of about 15 nm, so that these high-M polymers are coil-like in soln., rather than rigid rods. This persistence length is still expected to be several times larger than the effective conjugation length. [on SciFinder(R)]
The morphol. dependence of the photoluminescence (PL) properties of the pyridine-based polymers, poly(p-pyridylvinylene), poly(p-pyridine), and poly(p-pyridylvinylene-p-phenylenevinylene) (PPyVPV) was studied. The photoluminescence of soln. samples is characterized by high quantum efficiency (>70% in PPyVPV), weak coupling to vibrational modes (Huang-Rhys parameter ∼0.5) and a single-exponential decay (radiative lifetime ∼1 ns). On the other hand, film samples display strongly red shifted, featureless emission with low quantum yield (<20%) and highly nonexponential decay dynamics. Through consideration of absorption and excitation spectra, the \"site-selectivity\" of the PL, and the concn. dependence of the PL spectrum, we demonstrate that the red shifted film spectra are a result of the formation of low-energy aggregate sites due to strong interchain interactions. Time-resolved measurements suggest a longer radiative lifetime for the aggregate vs. soln., leading to the lower efficiency. Aggregate formation is morphol. dependent, and is minimal in \"powder\" samples which are pptd. after polymn. [on SciFinder(R)]
The authors present results of cw, time-resolved, and spatially resolved spectroscopic studies of emission and absorption in a model conjugated polymer, poly(p-pyridyl vinylene) (PPyV). The red shifted film spectra suggest the formation of aggregated regions. The ∼4X redn. in emission efficiency in films vs. soln. is attributed to a longer radiative lifetime for aggregate excitons, as is evidenced by time-resolved fluorescence measurements. The authors present direct optical imaging of aggregates in a conjugated polymer via near-field scanning optical microscopy. The aggregate emission and absorption are localized to partially aligned regions of the film ∼200 nm in size. [on SciFinder(R)]
We present results of picosecond photoinduced absorption (PA) and time-resolved photoluminescence studies on solid and soln. forms of poly(p-pyridylvinylene). The nearly identical PA response of all forms of the polymer reflects the generation of the same primary photoexcitation, a Couloumbically bound intrachain singlet exciton, and the absence of exotic species such as interchain excimers. The time dependence of the PA points to direct intersystem crossing as the origin of triplet excitons, ruling out generation of free carriers as a precursor to exciton formation. [on SciFinder(R)]
1984
Visnagin was isolated from M. divaricatum. Visnagin is known to alter the high d./low d. lipoprotein ratio in blood (Grammill, R. B. and Hyde, B. R., 1983). [on SciFinder(R)]
1985
The relation between the bulk modulus (K) and the coeff. of thermal expansion implied by the Murnaghan logarithmic equation of state are explicitly derived. Values for dK/dT can be obtained from thermal expansion measurements. These values are consistent with, and generally more accurate than, direct compressibility measurements. The equations are confirmed by calcns. for MgO AlO1.5, MgAl2O4, and MgSiO3. The temp. dependence of the bulk modulus can be predicted for solids for which there are no high-temp. compressibility measurements. [on SciFinder(R)]
A chem. investigation of M. divaricatum has resulted in the isolation of 19 coumarins, 5 of which are novel compds. and 18 are khellactone derivs. The coumarins were isolated from the crude exts. by a combination of adsorption chromatog., gel permeation and HPLC; the more successful HPLC sepns. utilized a nitrile bonded phase column. The structures were detd. by 1H NMR and mass spectral studies and by comparisons with literature data. The relative configuration for the entire series was secured from 1H NMR data, while the abs. configuration could be assigned with any certainty only in the cases of 2 of the compds. [on SciFinder(R)]
1986
Ring-opening metathetical polymn. of 3,4-diisopropylidenecyclobutene in the presence of titanocene methylidene [83876-46-4] catalysts yielded elec. conducting polymer which was sol. in C6H6 and CHCl3 in its undoped form and exhibited high elec. cond. in its doped form. Thermoelec. power measurements on I2-doped films showed that the charge carriers were pos. charged. [on SciFinder(R)]
1987
The polymer [104584-99-8] which is synthesized by ring opening olefin-metathesis polymn. of 3,4-diisopropylidenecyclobutene, is sol., transparent, and forms conductive materials (up to 200 $Ømega$-1cm-1) when doped with iodine. The characterization of the material by CP-MAS 13C NMR, ESR, and UV-vis spectroscopy is reported. The electronic structure and the possible mechanisms of conduction are discussed. [on SciFinder(R)]
1988
Polyacetylene (I) was prepd. by ring-opening metathesis polymn. of benzvalene in the presence of non-Lewis acidic W alkylidene catalysts to give polybenzvalene, followed by isomerization in the presence of HgCl2. I had low crystallinity and elec. cond. 1 s/cm upon doping with iodine. [on SciFinder(R)]
1989
The doping process in a recently developed conducting polymer precursor, poly(3,4-diisopropylidenecyclobutene) (PDPCB) was studied by ESR and optical spectroscopy. Thin films of PDPCB were exposed to several oxidants, I2, AsF5, or a combination of AsF5 and AsCl3 or AsF3, and monitored in situ by ESR. At the initial stages of doping, the films developed broad ESR spectra with a Gaussian line shape. Except in the case of doping with I2, resolved hyperfine structures from cation radicals were obsd. As the doping progressed, the ESR spectra gradually transformed to narrower line widths with a Lorentzian component. The Lorentzian component can be attributed to charge carrier species developed in the film through doping. The results of optical spectroscopy (UV-visible) are incorporated to elucidate the effect of doping on electronic transitions of the doped PDPCB. [on SciFinder(R)]
The synthesis and properties of the polybenzvalene and its conversion to polyacetylene are presented. This conversion is performed by treating polybenzvalene with Lewis acid catalysts. The highest quality material was obtained from the isomerization with HgCl2. The polyacetylene produced by this precursor route has a morphol. that is considerably more amorphous than other forms of polyacetylene that have been previously reported. Orientation of the precursor polymer by stretching induced crystallinity and chain alignment as detd. by x-ray diffraction. The unoriented polyacetylenes exhibited a cond. of 1 $Ømega$-1 cm-1 with I doping. Materials stretched to elongations of l/l0 = 2.3 and l/l0 = 6 displayed conductivities of 13 $Ømega$-1 cm-1 and 49 $Ømega$-1 cm-1, resp. Block copolymers of polynorbornene and polybenzvalene were produced. [on SciFinder(R)]
The synthesis and properties of the conductive polymer precursor polybenzvalene (I) and its conversion to polyacetylene (II) are presented. I was synthesized by ring-opening metathesis polymn. of benzvalene with non-Lewis acidic tungsten alkylidene catalysts. Treatment of I with solns. of HgCl2, HgBr2, ZnI2, and AgBF4 resulted in a C-C bond rearrangement and produced II. The II films produced with HgCl2 displayed the highest conductivities and the best mech. properties. The II produced was of low crystallinity and upon treatment with iodine exhibited conductivities of 1 S/cm. Oriented II was produced by stretching I and converting it to II. With iodine doping, the oriented II (6-fold stretching) displayed a cond. of 49 S/cm. [on SciFinder(R)]
1990
Polymers contg. quinone bisketal groups were synthesized by ring-opening metathesis polymn. of I and II. These polymers were investigated as sol. precursors to insol. conductive polymers. Hydrolysis produced the corresponding quinones which underwent tautomerization of hydroquinones. This process resulted in extended conjugation and elec. conduction. Thermolysis of quinone bisketals of 200° resulted in MeOH elimination to give red materials. Preliminary cond. studies of the doped hydrolyzed and thermolyzed polymers revealed conds. in the semiconductive region. [on SciFinder(R)]
1992
New discotic bimetallic liq. crystals, I, II, (r = (CH2)n
A series of liq. cryst. vanadyl 5-alkoxy-N,N'-disalicylidenediamine complexes are reported with ethylene (salen), propylene (salpn), and 2,2-dimethylpropylene (Me2salph) groups connecting the Schiff-base nitrogens. These compds. are under investigation as potential ferroelec. and second order nonlinear optical materials. The n(salen)VOs (n = no. of carbons in the alkoxy chain) are monomeric in the solid state and display highly disordered smectic A and smectic C structures as detd. by optical textures and x-ray powder diffraction. In the case of the (n(salpn)VO)s, the compds. exhibit a linear chain structure (e.g. (-V = O -V = O)n) in the solid state. For n(salpn)VO, the linear chain structure is too restrictive to allow a liq. cryst. state and these compds. melt at ∼300° with decompn. The n(Me2salpn)VOs also exhibit a linear chain structure as detd. by an x-ray crystal structure and IR spectroscopy, but steric repulsions weaken the linear chain structure sufficiently to allow melting into the liq. cryst. state. Variable temp. IR shows that the fluid liq. cryst. phase of these compds. exhibits a linear chain structure and becomes monomeric at temps. above their clearing point. X-ray powder diffraction and optical texture anal. show the thermotropic phases of the n(Me2salpn)VO complexes to display smectic structures. These results demonstrate that the n(Me2salpn)VO complexes comprise a new type of low-viscosity liq. cryst. polymer which assembles into a unidirectional linear chain structure. [on SciFinder(R)]
1993
The synthesis and ionochromic properties of polythiophenes functionalized with macrocyclic crown ether groups are described. These polymers are designed to be sensitive to alkali metal ions and chelation of the ion induces a twisting of the polymer's backbone from planarity. These conformational changes are of interest since they should ultimately lead to cond. changes which can be used to construct new sensory devices. Twisting of the polymer's backbone from planarity will also reduce the effective conjugation lengths, and polymers in which two of the crown ether oxygens are directly attached to the thiophene rings (I; X = O; x = 1, 2) exhibit large ionochromic responses which are easily detected visually. Only small responses are detectable for I (X = CH2O; x = 1, 2) which exhibit smaller assocn. consts. The large ionochromic responses are mainly the result of twisting at a no. of sites along the polymer chain; however electrostatic factors may also play a role. The ion selectivity is similar to that obsd. for simpler crown ether macrocycles and the smaller rings give the largest response for Na+ ions and the larger rings are most sensitive to K+. [on SciFinder(R)]
This report demonstrates the interrelationships between liq. cryst. superstructure and the strength of linear chains (–V-O...V = O–)n, exhibited by vanadyl-based discotic liq. crystals. The effect of mol. superstructure was detd. by studying related compds. which due to their shapes display liq. crystal phases with specific intermol. correlations between nearest neighbors. In superstructures which do not exhibit steric interactions, the strength of assocn. in the linear chain structure was controlled by the groups bridging between the Schiff-base nitrogens. Compds. with bridging group a are monomeric, compds. with bridging group b are the strongest linear chains, and compds. with bridging group c are somewhat weaker linear chains. The relative strength of the linear chains was detd. by monitoring the V=O stretching frequency and is sensitive to mesophase superstructures which produced specific steric repulsions and/or orientational preferences. Linear chain structures also influence the nature of the mesophases and produced novel polar mesophases with high intercolumnar order yet liq.-like order within the columns. This increased intercolumnar order is ascribed to an increase in the rigidity of the linear chain which holds the mesogens in a tighter registry. [on SciFinder(R)]
New bowl-shaped (bowlic) liq. crystals with rigid W-oxo calix[4]arene (I; Z = N:NC6H2-3,4-(O(CH2)12H)2-5-R where R = H, O(CH2)12H) based mesogens 1a and 1b, resp., are reported. These compds. exhibit discotic columnar phases which are stable over approx. a 200° temp. range. The uncomplexed tetra-phenol ligand displays only a transient mesophase on the first heating, and the conformational rigidity provided through W-oxo complexation is necessary for well behaved mesomorphism. For 1a, the clearing point is at 320° which leads to decompn. in the isotropic phase. The addn. of 4 more sidechains to form 1b results in a lower clearing point (267°) and a stable isotropic phase. Polarized optical microscopy and DSC indicate that 1b displays a bowlic Dho phase (also known as PA or Bho), whereas studies on 1a's mesophase were less definitive. Both 1a and 1b exhibit a pronounced tendency to bind Lewis base guests in their cavities, and DMF forms very strong complexes which were spectroscopically characterized. The DMF guest produces large effects on the phase behavior by suppressing mesomorphism and lowering the isotropic points of 1a and 1b to 115 and 84°, resp. This extreme sensitivity of the DMF guest suggests that the mols. organize in head-to-tail structures in the mesophases and that the W-oxo group of one mol. protrudes into the cavity of the neighboring mol. [on SciFinder(R)]
The dependence of mesomorphism of 1,3-diketonate V-oxo (vanadyl) complexes on the no. of side chains was studied. These complexes have a large dipole normal to the disk plane and are under study in an effort to generate discotic phases with polar order. Relatively complex phase behavior is obsd. when the vanadyl 1,3-diketonate complexes are appended with 4 chains. These complexities are most likely related to the order/disorder assocd. chains were prepd. by replacement of 2 of the Ph groups with Me or trifluoromethyl groups, and these complexes displayed only crystal phases. Complexes with 2 trialkyloxy phenyls and 2 dialkyloxy phenyls were synthesized and found to display a very stable Dhd phase. The presence of addnl. alkyloxy groups was found also to promote a linear chain structure, (i.e.-V=O–V=O–), in the crystal phase. [on SciFinder(R)]
1994
A review, with 10 refs., is given on the design of conducting polymeric sensory materials. They exhibit ionochromic, electrochem., or resistive responses to specific chem. signals. The integration of mol. recognition elements into polymers of pyrrole, thiophene, and bithiophene is described. [on SciFinder(R)]
A study was made of ferroelec. liq. crystals from conical MoO2 complexes with polar polymer ligands. A hexagonal columnar liq. crystal phase is obsd. The heats of transition are given. A method for internal stabilization of the polar superstructure is described. [on SciFinder(R)]
A comprehensive study of the liq.-cryst. properties of 51 bimetallic compds. based upon 1,3,5-triketonate and 1,3,5,7-tetraketonate ligands is reported. These materials are liq. cryst. when six or more side chains are appended to the mesogenic core, and only columnar phases were obsd. Most of the liq. crystals were homonuclear dicopper complexes. Schiff-base derivs. of some of the triketones allowed for the synthesis of heteronuclear bimetallic liq. crystals. The NiCu and NiPd Schiff-base complexes are the 1st heteronuclear liq. crystals with proximate (strongly interacting) metal centers. Other heteronuclear complexes studied were not liq. cryst. due to the tendency to retain coordinated solvent or to form strongly assocd. structures in the absence of axial ligands. The use of complementary shapes was demonstrated as a means to generate av. relative organizations (correlations) between the complexes. The presence of these correlated structures was shown through comparisons of the structures, phase behavior, and the immiscibility between materials having the same phase but different shapes. Correlated structures were shown which produce av. rotations of 90° and 180° between nearest-neighbor mols. A crystal structure of one compd. confirmed that a similar superstructure was exhibited in the solid state. The correlated structures exhibit relatively short (3.29 \AA) correlations between the mesogens, thereby allowing for strong intermol. interactions. The ability to control the orientation and relative position of transition metal centers in liq. crystals has applications in the design of new liq.-cryst. materials with useful magnetic and electronic properties. [on SciFinder(R)]
New correlated columnar liq. crystals which are based upon square-planar 1,3-diketonate Schiff-base complexes of Pd, Ni, and Cu were developed. These compds. exhibit a no. of desirable liq.-cryst. properties such as ease of alignment, low m.ps., and low viscosity. The complexes are correlated into a discotic antiphase structure in which the complexes are oriented antiparallel. This structure facilitates very close intermesogen contacts of 3.6 \AA since the antiphase structure directs the sterically bulky side chains away from those of the nearest neighbors. Vanadyl complexes are not liq. crystals. [on SciFinder(R)]
A review with 18 refs. of bowl-shaped (bowlic) liq. crystals of calixarenes and tungsten-oxo calix[4]arenes. [on SciFinder(R)]
A review with 22 refs. primarily on the authors' work, discussing the use of transition metal-catalyzed coupling reactions in the synthesis of conducting thiophene-based and ladder polymers. [on SciFinder(R)]
Diacetylene macrocycles I (R = H, C4H9, C6H13, C10H21, C12H25, OC10H21; n= 1-3) were prepd. from the oxidative coupling of 1,2-diethynylbenzene derivs. I can be produced in useful quantities and are of interest as precursors to novel conjugated org. polymers. The reported results indicate that when the R groups are large the dimeric macrocycle (n = 1) can be prepd. in as high as 74% yield from the corresponding 1,2-diethynylbenzene in a 1-step procedure. An alternate multistep procedure was found to produce the tetrameric macrocycle I (n =
The synthesis and electrochem. properties of the macrocycle-contg. polythiophene I are described. I forms a self-assembled pseudopolyrotaxane in the presence of $π$-deficient guests such as paraquat and 1,1'-bis(4-fluorobenzyl)-2,2'-bipyridyl. Pseudopolyrotaxane formation results in both an anodic shift in the oxidn. potential of the polymer as well as a significant decrease in its max. cond. (ca. 52% at 45 mM guest). This effect is completely reversible. For comparative purposes, a nonmacrocyclic model polymer was also studied and showed no significant change in max. cond. under identical conditions. The results clearly demonstrate that the chemoresistive response is a direct result of host-guest complexation. Such a chemoresistor approach is an attractive route into conducting polymer-based sensors wherein resistivity is attenuated by perturbations arising from host-guest interactions. [on SciFinder(R)]
The development of a novel reaction for the synthesis of fused polycyclic benzenoid arom. hydrocarbons is reported. In the reaction's simplest form, the attack of an electrophile on a substituted 2-(ethynyl)biphenyl would provide a substituted phenanthrene. To demonstrate the utility of the reaction the authors have synthesized in all carbon conjugated ladder polymer or graphite ribbon. En route to this target, model systems of the general structure 2',5'-di(4-alkoxyphenylethynyl)-p-terphenyl were synthesized for characterization and optimization purposes. Cyclization to give substituted dibenz[a,h]anthracenes consistently proceeded in very high to quant. yield. The graphite ribbon polymer, a yellow-orange material, was synthesized by TFA treatment of the colorless poly[2,5-di(dodecyl)-2',5'-di(4-(dodecyloxy)phenylethynyl)-p-biphenylene]. All compds. were characterized using 1H and 13C NMR, IR, UV-VIS and luminescence spectroscopy, high resoln. mass spectrometry, and elemental anal. [on SciFinder(R)]
A new class of metallomesogens contg. $\beta$-diketone derivs. with octahedral structures are described. The observation of liq. crystallinity in these materials was unexpected as a result of their low aspect ratios. To better understand these materials, the dependence of the mesomorphism on the no. of sidechains (N) and metal center was investigated. [on SciFinder(R)]